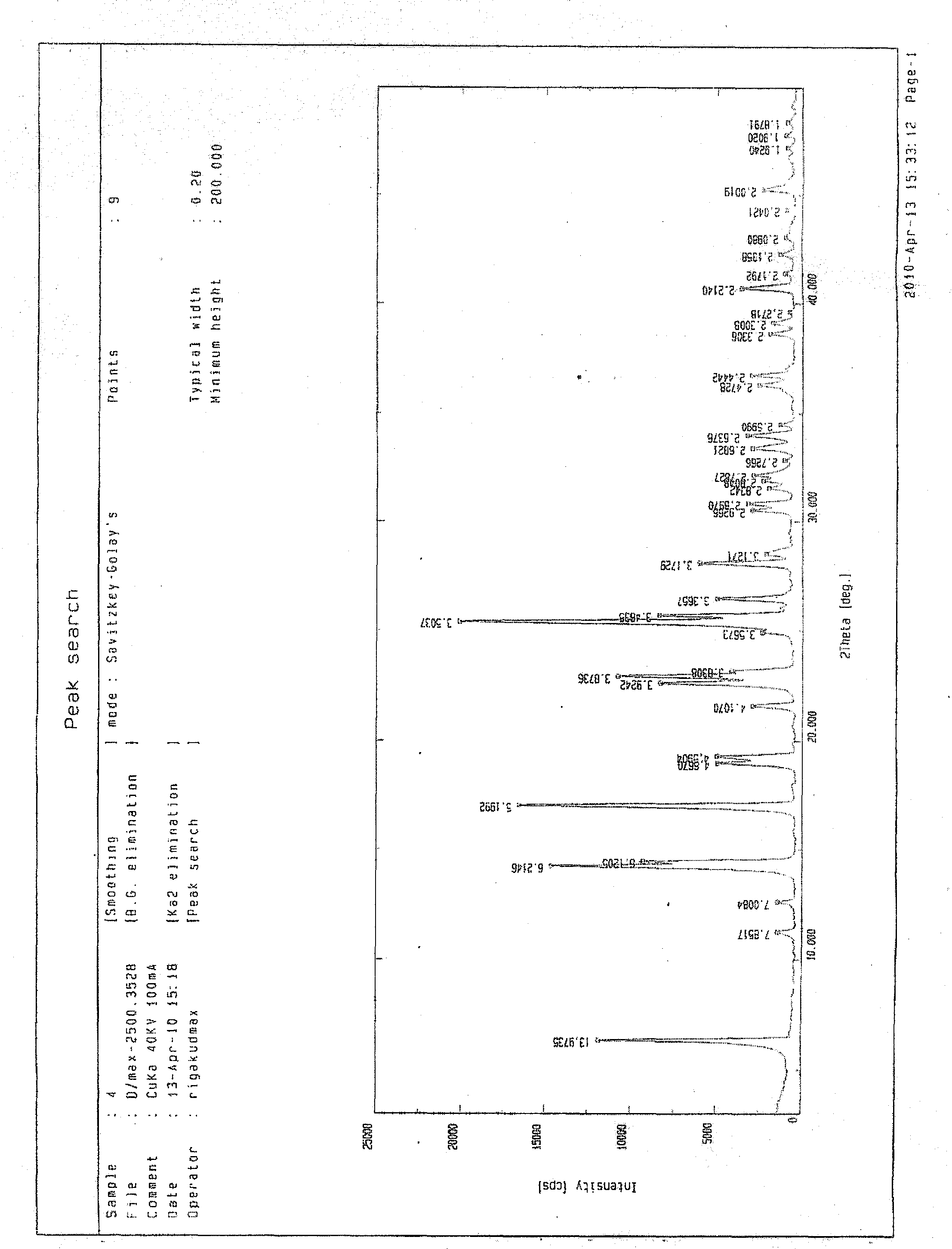
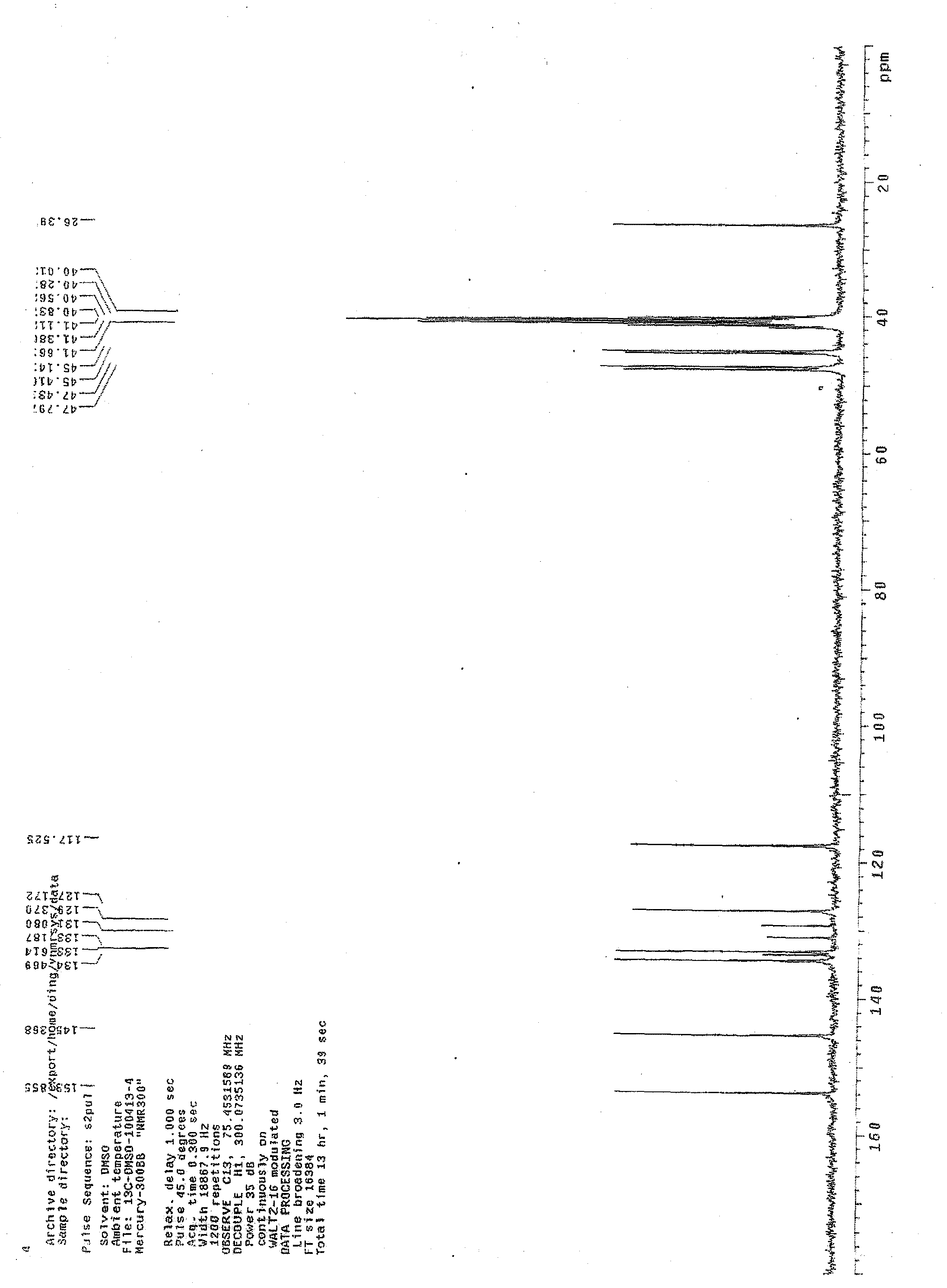
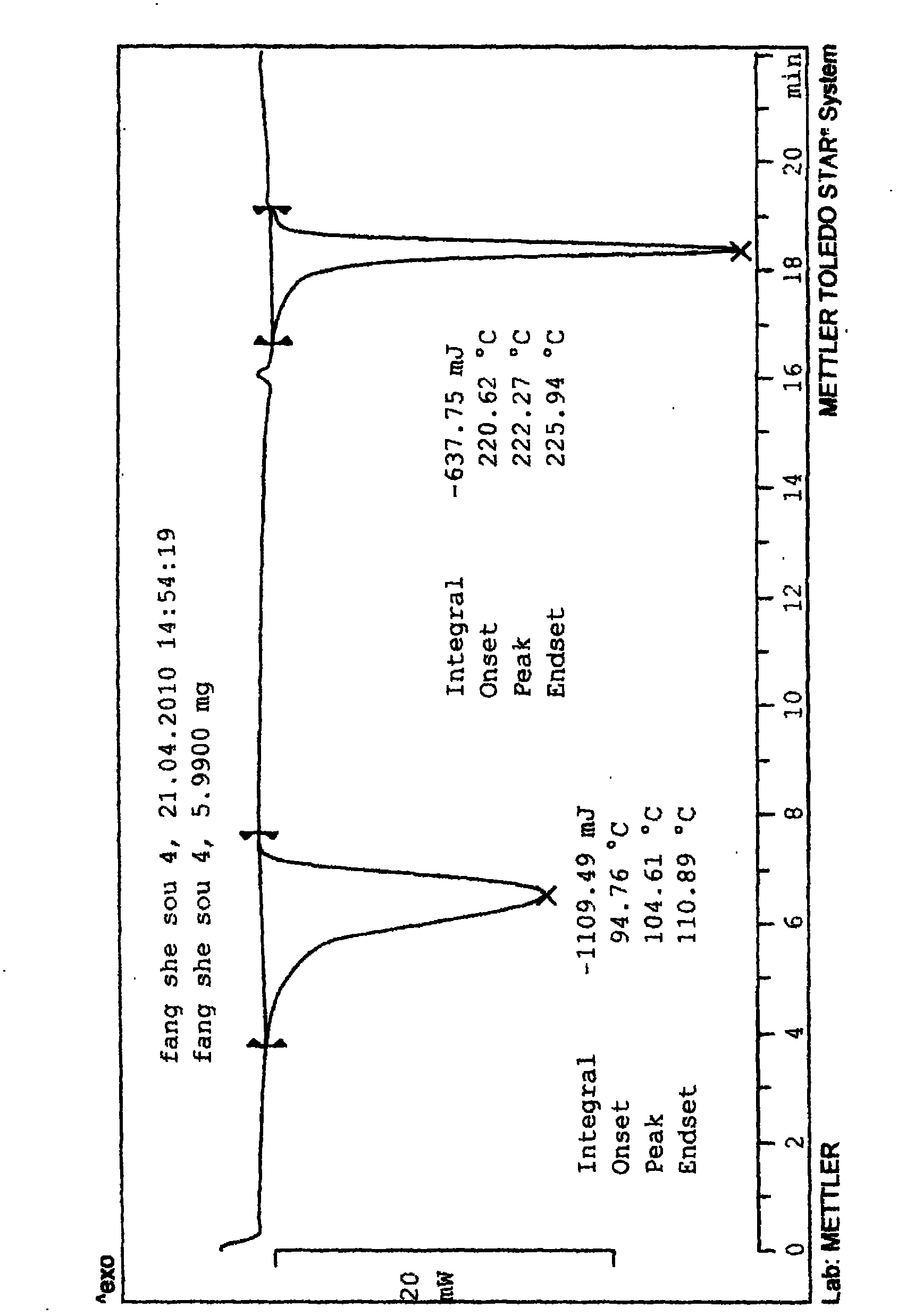
Fasudil crystal formation IV as well as preparation method and application thereof
A technology of fasudil hydrochloride and its crystal form, which is applied in the field of medicine and can solve problems such as flammability, unfriendly smell of n-butanol, and easy volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1200mL of the dichloromethane solution of the substituent of 5-sulfonylchloroisoquinoline and homopiperazine contains about 200g of the substituent. After being decolorized by activated carbon, salted with hydrochloric acid, extracted and separated, 500mL of light yellow aqueous phase was separated, which contained Fasudil hydrochloride.
Embodiment 2
[0042] Get the aqueous phase solution obtained in Example 1, pour it into a 3L round-bottomed flask, add 1000mL of isopropanol, heat, start stirring, when the solution temperature reaches about 65°C, start vacuum distillation, along with the separation of isopropanol and water After the azeotrope is evaporated, the volume of the solution is continuously reduced, and then 500mL of isopropanol is added. When the aqueous solution is about 600mL, a large amount of solids appear. Cool naturally, filter at 25°C, and dry at 80°C to obtain Fasan hydrochloride Dier crystals.
Embodiment 3
[0044] Get the aqueous phase solution obtained in Example 1, pour it into a 3L round-bottomed flask, add 1000mL of isopropanol, heat, start stirring, when the solution temperature reaches about 65°C, start vacuum distillation, along with the separation of isopropanol and water After the azeotrope is evaporated, the volume of the solution is continuously reduced, and then 500mL of isopropanol is added. When the evaporated aqueous solution is about 600mL, a large amount of solids appear. Cool naturally, filter at 55°C, and dry at 80°C to obtain Fasan hydrochloride Dier crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com