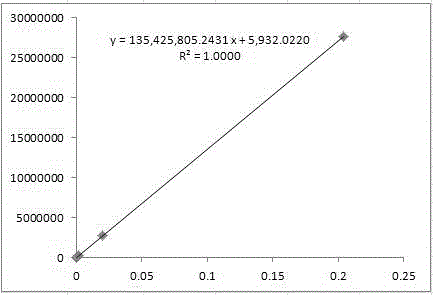
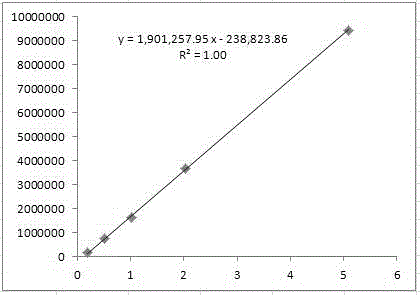
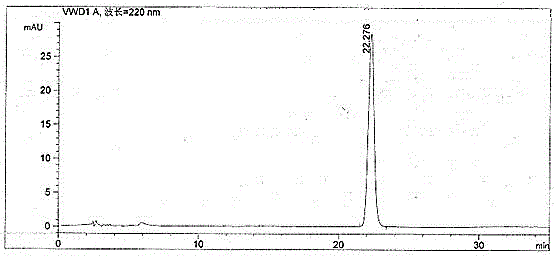
Quality control method for fasudil hydrochloride
A technology of fasudil hydrochloride and a quality control method, applied in the field of quality control of fasudil hydrochloride, can solve the problem of quality detection and control of dimer and homopiperazine, influence the production and quality assurance of fasudil hydrochloride , can not completely effectively and accurately control the quality of fasudil hydrochloride and other problems to achieve the effect of improving product quality, high precision and accuracy, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Synthesis of 1,4-bis(5-isoquinolinesulfonyl)-pentahydro-1,4 diazepine dimer
[0037] Add 50.0g of isoquinoline-5-sulfonyl chloride hydrochloride and 150ml of dichloromethane into a 500ml reaction flask, mix and cool to 0°C, dropwise add aqueous sodium bicarbonate (16.5g / 250mlH 2O) adjust the pH to 5, then keep stirring for 25min, let stand for stratification, separate the dichloromethane layer, extract the water layer with dichloromethane, combine the organic layers, and dry with anhydrous magnesium sulfate at a temperature of 0°C to obtain a dry solution ;
[0038] Dissolve 18.8g of homopiperazine in 150ml of anhydrous tetrahydrofuran, add dropwise to the dry solution at 0°C, keep stirring for 1.5h, adjust the pH to 5, stir for 1h, concentrate the reaction solution to dryness under reduced pressure, add 500ml of water, stirred at 50°C for 2h, filtered and collected the filter cake, took a 500ml flask, added the above solid, added 250ml of methanol, heated t...
Embodiment 2
[0040] Example 2: Synthesis of 1,4-bis(5-isoquinolinesulfonyl)-pentahydro-1,4 diazepine dimer
[0041] Add 50.0g of isoquinoline-5-sulfonyl chloride hydrochloride and 150ml of dichloromethane into a 500ml reaction flask, mix and cool down to 5°C, dropwise add aqueous sodium bicarbonate (16.5g / 250mlH 2 O) adjust the pH to 6, then keep stirring for 35 minutes, let stand to separate the layers, separate the dichloromethane layer, extract the water layer with dichloromethane, combine the organic layers, and dry with anhydrous magnesium sulfate at a temperature of 5°C to obtain a dry solution ;
[0042] Dissolve 18.8g of homopiperazine in 150ml of anhydrous tetrahydrofuran, add dropwise to the dry solution at 5°C, keep stirring for 2.5h, adjust the pH to 5, stir for 1h, concentrate the reaction solution to dryness under reduced pressure, add 500ml of water, stirred at 50°C for 2h, filtered and collected the filter cake, took a 500ml flask, added the above solid, added 250ml of met...
Embodiment 3
[0044] Example 3: Synthesis of 1,4-bis(5-isoquinolinesulfonyl)-pentahydro-1,4 diazepine dimer
[0045] Add 50.0g of isoquinoline-5-sulfonyl chloride hydrochloride and 150ml of dichloromethane into a 500ml reaction flask, mix and cool to 3°C, dropwise add aqueous sodium bicarbonate (16.5g / 250mlH 2 O) Adjust the pH to 5.5, heat and stir for 28 minutes, let stand to separate the layers, separate the dichloromethane layer, extract the water layer with dichloromethane, combine the organic layers, and dry with anhydrous magnesium sulfate at a temperature of 2°C to obtain a dry solution ;
[0046] Dissolve 18.8g of homopiperazine in 150ml of anhydrous tetrahydrofuran, add dropwise to the dry solution at 3°C, keep stirring for 2 hours, adjust the pH to 5, stir for 1 hour, concentrate the reaction solution to dryness under reduced pressure, add 500ml water, stir at 50°C for 2 hours, filter and collect the filter cake, take a 500ml flask, add the above solid, add 250ml of methanol, hea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com