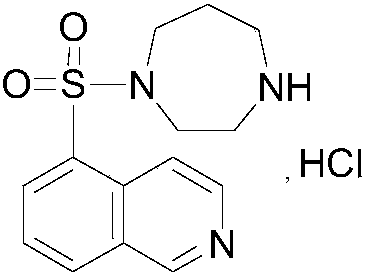
Method for determination of content of related impurity (5-methyl isoquinolinesulfonic acid) of fasudil hydrochloride
A technology of methyl isoquinolinesulfonate and fasudil hydrochloride, which is applied in the field of high-performance liquid chromatography analysis and detection of fasudil hydrochloride impurity 5-isoquinolinesulfonate methyl ester, can solve the problem of 5-isoquinolinesulfonate Solvent problems such as weak absorption, non-detection, and poor separation effect of methyl phenol sulfonate, to achieve the effects of good stability and reproducibility, high sensitivity, high accuracy and precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Experimental Equipment and Reagents
[0032] (1) Experimental equipment:
[0033] Instrument: Shimadzu 20A with UV detector
[0034] (2) Chromatographic conditions:
[0035] Chromatographic column: ACCHROM XAmide column, 4.6×250mm, 5um;
[0036] Flow rate: 0.9~1.1ml / min;
[0037] The detection wavelength is 238nm;
[0038] Column temperature: 28~32℃;
[0039] Injection volume: 20ul;
[0040] mobile phase:
[0041] A: 0.05mol / l ammonium dihydrogen phosphate aqueous solution;
[0042] B: Methanol;
[0043] A:B=(91~94):(6~9)(V:V)
[0044] (3) Experimental reagents:
[0045] Methanol: Beijing Bailingwei Technology Co., Ltd., HPLC grade;
[0046] Ammonium dihydrogen phosphate: Sinopharm chemical reagent, analytical grade
[0047] Implementation steps:
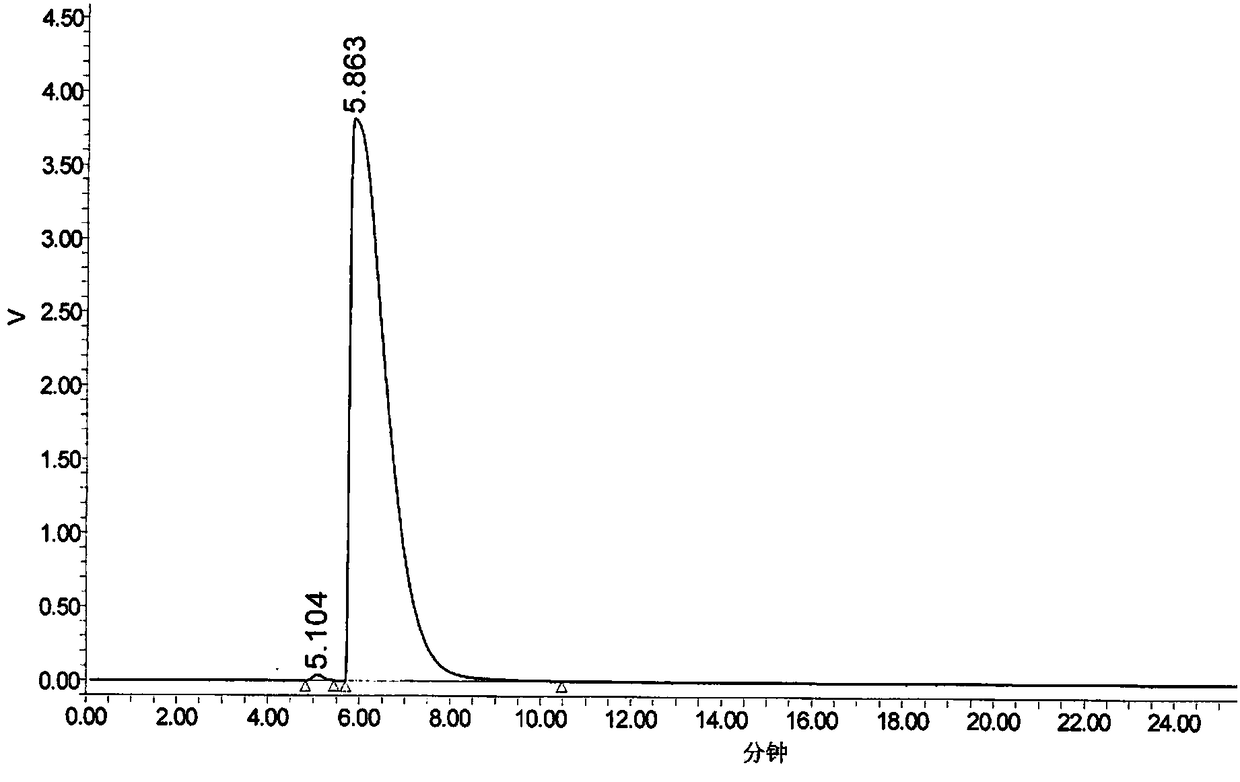
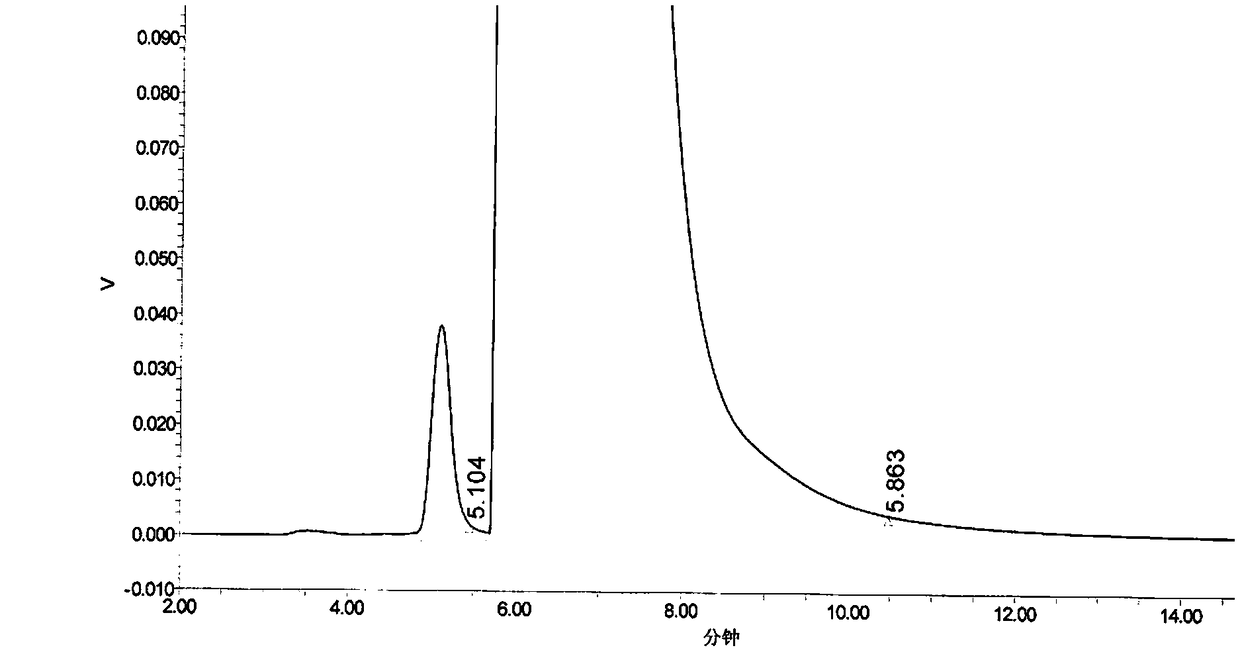
[0048] 1. Precisely measure 20 μl of the mobile phase, inject it into the liquid chromatograph, and record the chromatogram; the result has no interference with the determination of impurities. Accurately weigh ...
Embodiment 2
[0051] Experimental Equipment and Reagents
[0052] (1) Experimental equipment:
[0053] Instrument: Shimadzu 20A with UV detector
[0054] (2) Chromatographic conditions:
[0055] Chromatographic column: ACCHROM XAmide column, 4.6×250mm, 5um;
[0056] Flow rate: 1.0ml / min;
[0057] The detection wavelength is 238nm;
[0058] Column temperature: 30°C;
[0059] Injection volume: 20ul;
[0060] Mobile phase: 0.05mol / l ammonium dihydrogen phosphate aqueous solution:methanol=92:8(V:V)
[0061] (3) Experimental reagents:
[0062] Methanol: Beijing Bailingwei Technology Co., Ltd., HPLC grade;
[0063] Ammonium dihydrogen phosphate: Sinopharm chemical reagent, analytical grade
[0064] Implementation steps:
[0065] Take about 250mg of Fasudil hydrochloride, weigh it accurately, put it in a 25ml measuring bottle, add 20ml of methanol to dissolve, then accurately add 1.0ml of impurity standard stock solution, dilute to the mark with methanol, shake well, and use it as a precis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com