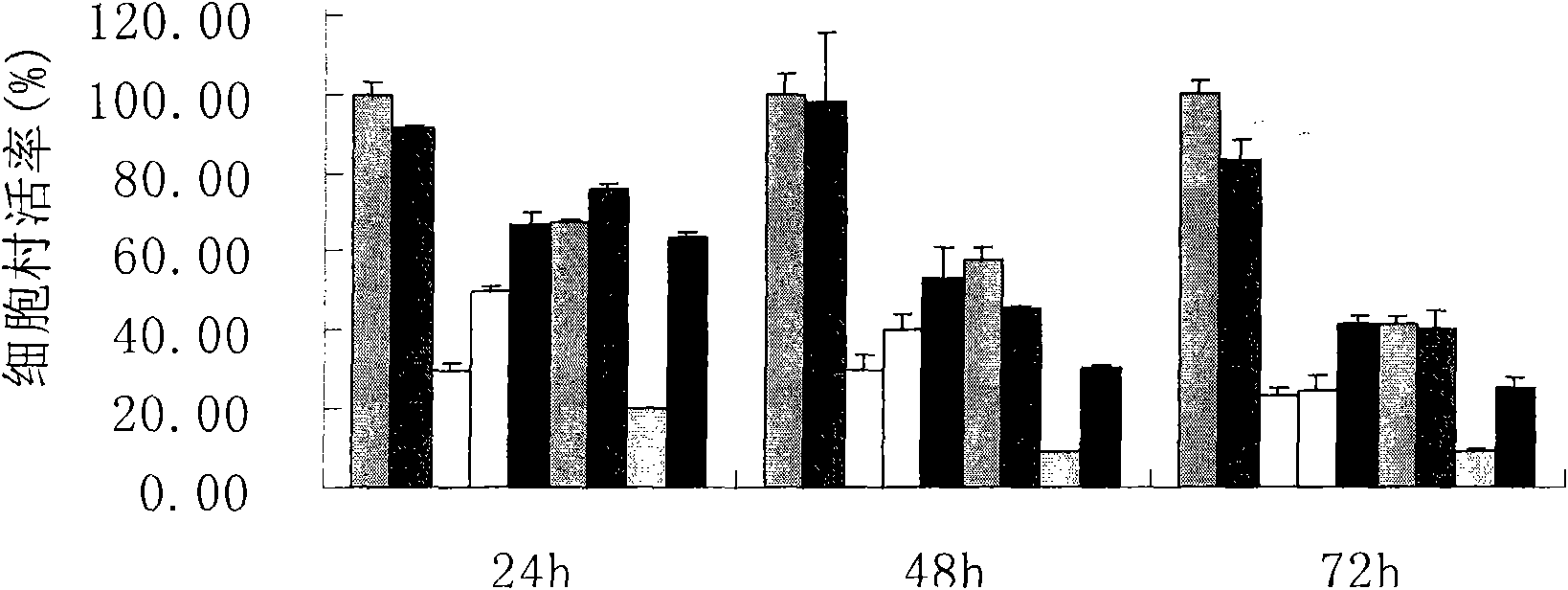
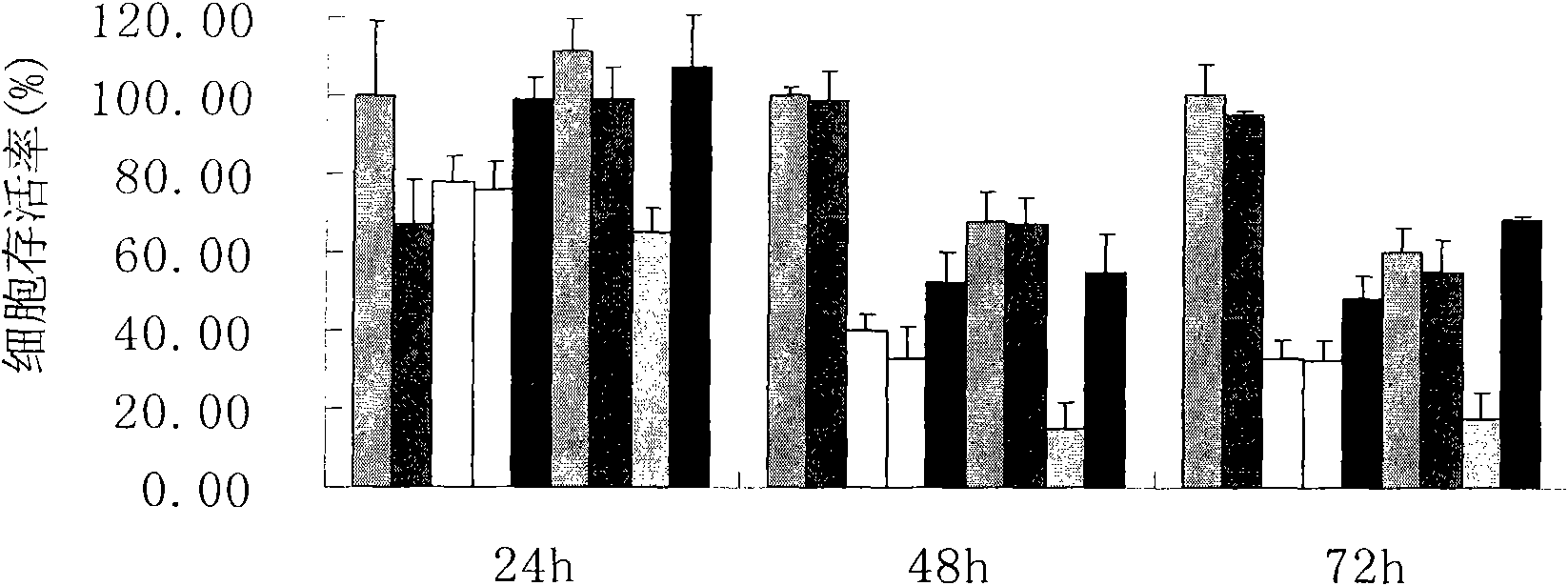
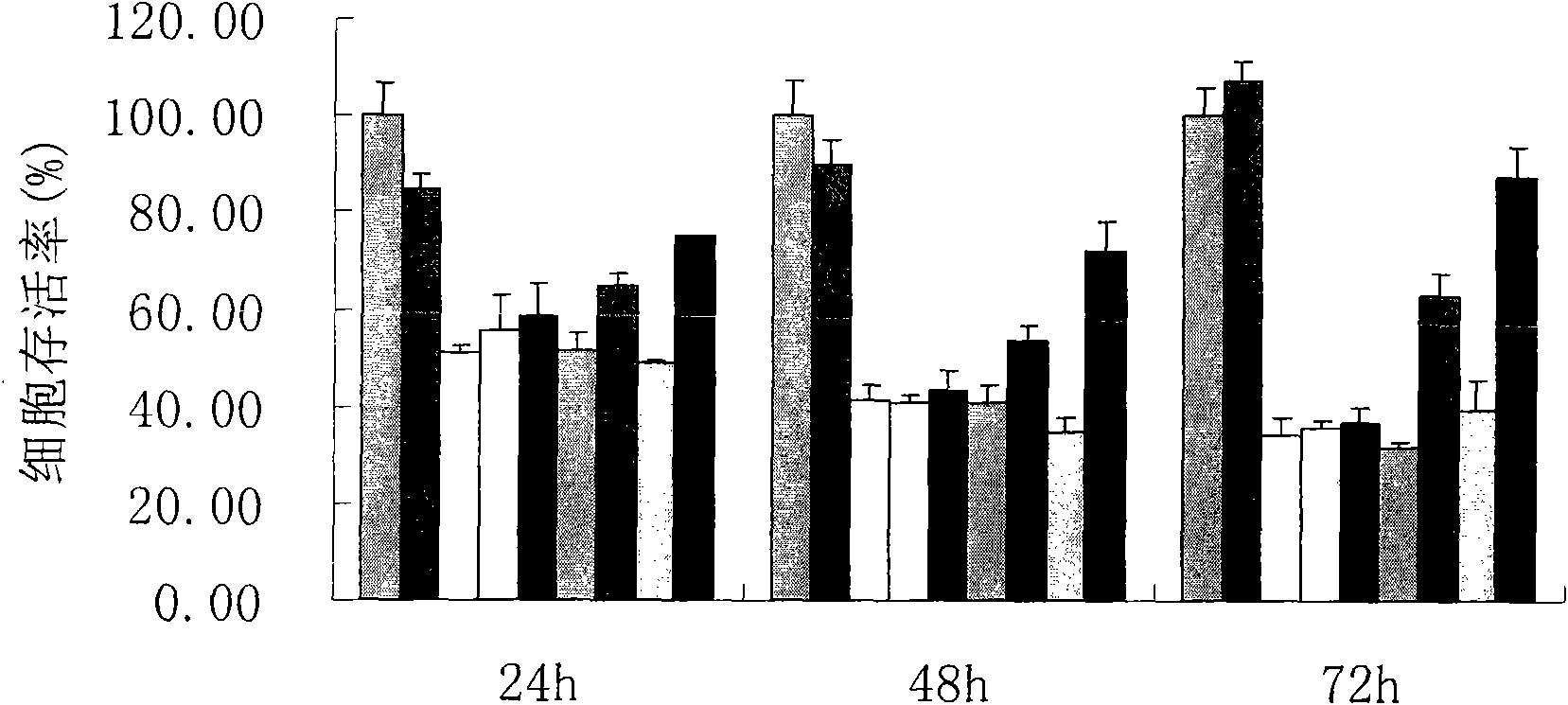
Piperazidine or homopiperazine oxalyl hydrazine class compound, preparation and use thereof
A compound, selected technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve the problem of incomplete research on the relationship between the structure and properties of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0213] Example 1, (4-benzyl-[1,4]diazepan-1-yl)-oxo-acetyl (3-allyl- Preparation of 2-hydroxy-methylenephenyl)hydrazine and its salt
[0214] The reaction scheme is outlined as follows:
[0215]
[0216]
[0217] step 1. Under the condition of 0 ℃ and electromagnetic stirring, in the reaction flask equipped with 1.9 g (10 mmol) of 4-benzyl homopiperazine and 1.11 g (11 mmol) of triethylamine and 50 ml of toluene, A solution of 2.0 g (15 mmol) ethyl oxalyl chloride in dichloromethane was added dropwise. After completion of the dropwise addition, stir at room temperature for 8 hours. After the reaction, add 50 ml of water, separate the organic layer, and use Na 2 SO 4 dry. After filtration and concentration, the obtained product was directly used in the next reaction with a yield of 95%.
[0218] Step 2. 2.94 g (10 mmol) of 4-benzyl homopiperazine oxaloethyl ester was added to a 300 ml round bottom flask, and 0.56 g (15 mmol) of 85% hydrazine hydrate was slowly...
Embodiment 2
[0221] Embodiment 2, (4-benzyl-piperidin-1-yl)-oxo-acetyl (2-hydroxyl-methylene phenyl) hydrazine and its salt preparation
[0222] The reaction scheme is outlined as follows:
[0223]
[0224] step 1. Under the condition of 0 ℃ and electromagnetic stirring, in the reaction flask equipped with 1.75 g (10 mmol) of 4-benzylpiperidine and 1.11 g (11 mmol) of triethylamine and 50 ml of toluene, gradually A dichloromethane solution of 2.0 g (15 mmol) ethyl oxalyl chloride was added dropwise. After completion of the dropwise addition, stir at room temperature for 8 hours. After the reaction, add 50 ml of water, separate the organic layer, and use Na 2 SO 4 dry. After filtration and concentration, the obtained product was directly used in the next reaction with a yield of 85%.
[0225] Step 2. 2.75 g (10 mmol) of 4-benzylpiperidine-1-oxaloethyl ester was added to a 300 ml round bottom flask, and 0.56 g (15 mmol) of 85% hydrazine hydrate was slowly added dropwise under ...
Embodiment 3
[0228] Example 3, (pyridine-3-methyl-piperazin-1-yl)-oxo-acetyl (3,5-dichloro-2-hydroxyl- Preparation of methylphenyl) hydrazine and its salt
[0229] The reaction scheme is outlined as follows:
[0230]
[0231] step 1. Under the condition of 0 ℃ and electromagnetic stirring, add 1.77 g (10 mmol) of [4-(1-pyridine-3-methyl)] piperazine- and 1.11 g (11 mmol) of triethylamine In a reaction flask with 50 ml of toluene, 2.0 g (15 mmol) of monoethyl oxalyl chloride in dichloromethane was added dropwise. After completion of the dropwise addition, stir at room temperature for 8 hours. After the reaction, add 50 ml of water, separate the organic layer, and use Na 2 SO 4 dry. After filtration and concentration, the obtained product was directly used in the next reaction with a yield of 90%.
[0232] Step 2. [4-(1-pyridine-3-methyl)]piperazine-1-oxalyl ethyl ester of 2.77 grams (10 mmoles) was added in the round-bottomed flask of 300 milliliters, slowly dripped 0.56 gram...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com