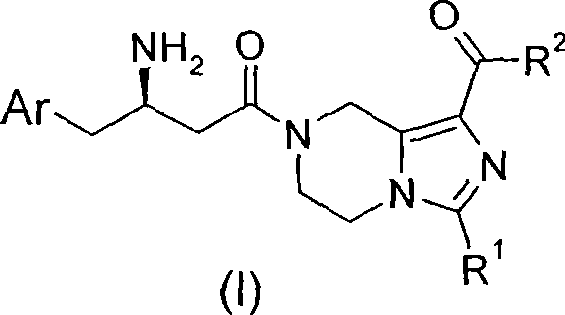
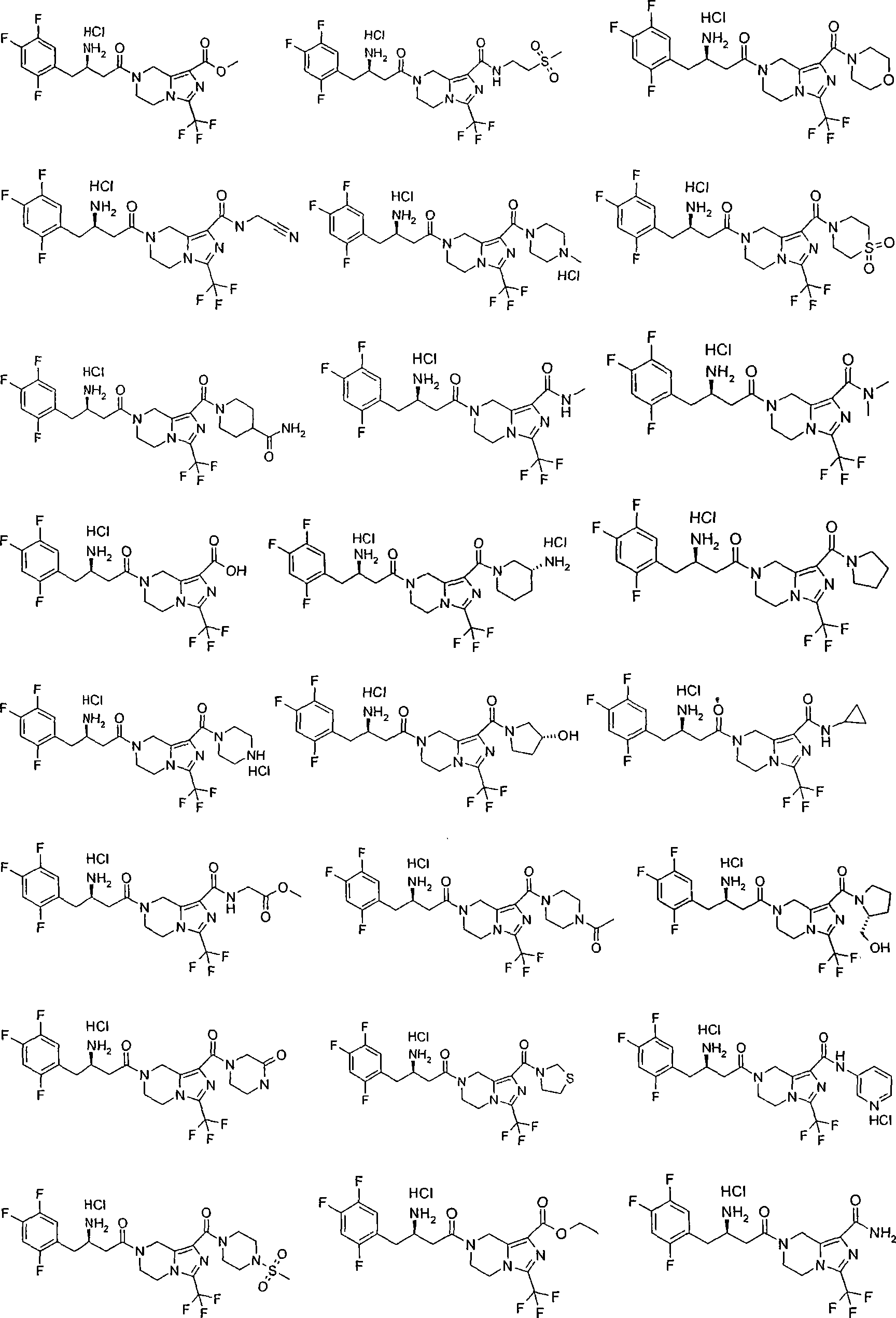
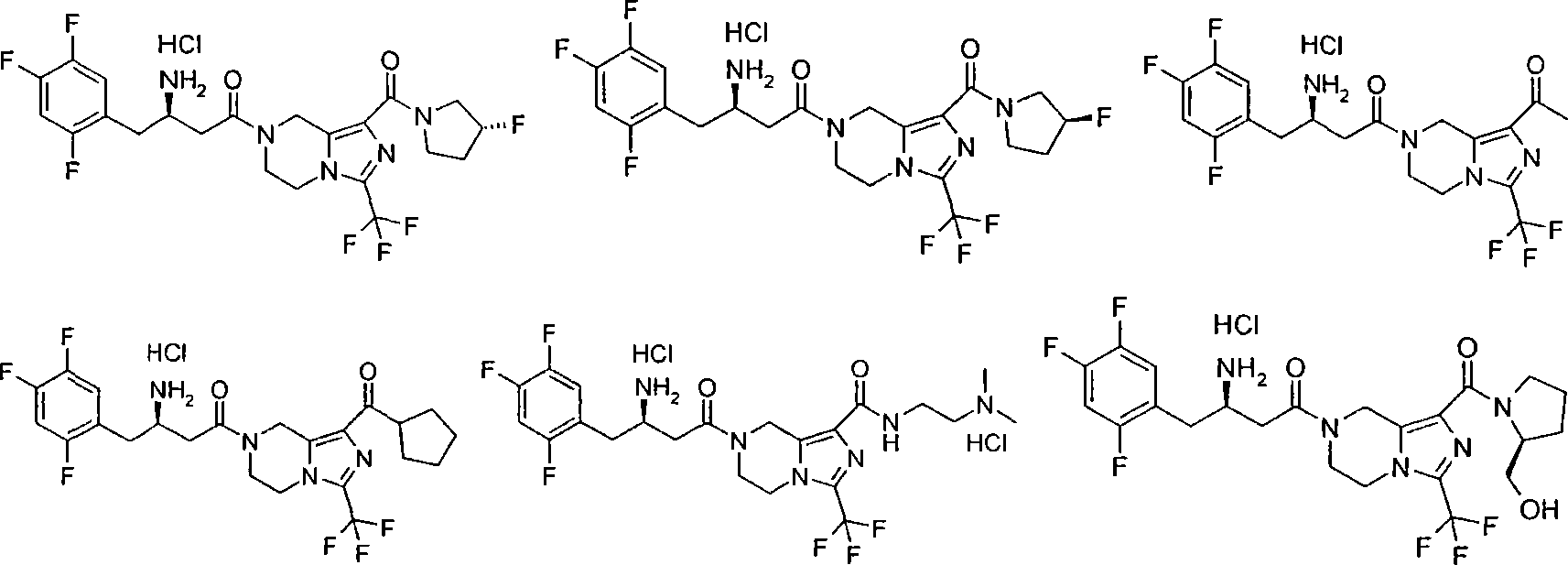
Piperazine derivative, preparation thereof and use thereof in medicine
A kind of pharmacy and compound technology, applied in the field of dipeptidyl peptidase IV inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] (R)-7-[3-amino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazole Methyl [1,5-a]pyrazine-1-carboxylate hydrochloride
[0122]
[0123]
[0124] first step
[0125] 2,2-Dimethyl-5-[2-(2,4,5-trifluoro-phenyl)-acetyl]-[1,3]dioxane-4,6-dione
[0126] 2,2-Dimethyl-[1,3]dioxane-4,6-dione (5.69g, 39.5mmol) was dissolved in 400mL dichloromethane under stirring, and 2 was added under ice-cooling, 4,5-Trifluorophenylacetic acid 1a (7.15g, 37.6mmol) and p-dimethylaminopyridine (7.35g, 60.2mmol), slowly add 250mL of 1-(3-dimethylamino-propyl)-3-ethyl Base-carbodiimide hydrochloride (8.28g, 43.2mmol) dichloromethane suspension, stirred at room temperature for 36 hours, with 5% potassium bisulfate solution (250mL × 7) and saturated sodium chloride solution ( 250mL×2) Wash the reaction solution, dry it with anhydrous magnesium sulfate, filter it with suction, and concentrate the filtrate under reduced pressure to obtain the title product 2,2-di...
Embodiment 2
[0179] (R)-7-[3-amino-4-(2,4,5-trifluorophenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo [1,5-a]pyrazine-1-carboxylic acid (2-methylsulfonyl-ethyl)-amide hydrochloride
[0180]
[0181] first step
[0182] (R)-7-[3-tert-butoxycarbonyl-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetra Hydrogen-imidazo[1,5-a]pyrazine-1-carboxylic acid
[0183] (R)-7-[3-tert-butoxycarbonylamino-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8 -Tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid methyl ester 1n (2.0g, 3.5mmol) was dissolved in 50mL methanol under stirring, added 30mL4N sodium hydroxide solution, and reacted at room temperature for 1 Hours later, follow the reaction by thin-layer chromatography, the raw materials disappeared, add 2N hydrochloric acid to adjust the pH of the reaction solution to about 3, extract the reaction solution with ethyl acetate (50mL×4), combine the organic phases, dry with anhydrous magnesium sulfate, and fi...
Embodiment 3
[0195] (R)-3-Amino-1-[1-(morpholine-4-carbonyl)-3-trifluoromethyl-5,6-dihydro-8H-imidazo[1,5-a]pyrazine- 7-yl]-4-(2,4,5-trifluorophenyl)-butan-1-one hydrochloride
[0196]
[0197] first step
[0198] (R)-[3-[1-(morpholine-4-carbonyl)-3-trifluoromethyl-5,6-dihydro-8H-imidazo[1,5-a]pyrazin-7-yl ]-3-oxo-1-(2,4,5-trifluoro-benzyl)-propyl]-tert-butyl carbamate
[0199] (R)-7-[3-tert-butoxycarbonyl-4-(2,4,5-trifluoro-phenyl)-butyryl]-3-trifluoromethyl-5,6,7,8- Tetrahydro-imidazo[1,5-a]pyrazine-1-carboxylic acid 2a (60 mg, 0.109 mmol), morpholine (19 mg, 0.218 mmol) and bis(2-oxo-3-oxazolidinyl) Phosphinoyl chloride (53.5mg, 0.218mmol) was dissolved in 5mL of dichloromethane with stirring, triethylamine (0.1mL, 0.65mmol) was added, and reacted overnight at room temperature. The reaction was tracked by thin-layer chromatography. The raw materials basically disappeared, and the reaction was evaporated to dryness. liquid, and the resulting residue was purified by silica gel colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com