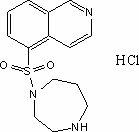
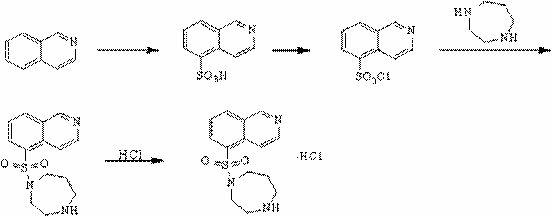
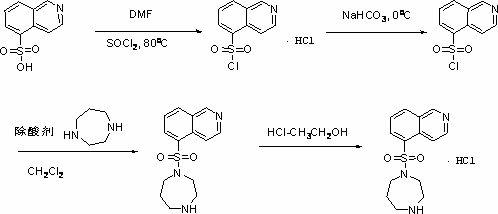
Synthesis and preparation method of fasudil hydrochloride
A technology for fasudil hydrochloride and isoquinoline sulfonyl chloride hydrochloride, applied in the field of medicine, can solve problems such as unpublished yield, and achieve the effects of reducing production cost and usage amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] A kind of synthetic and preparation method of Fasudil hydrochloride, this preparation method comprises the steps:
[0024] (1) In the presence of catalyst N,N-dimethylformamide, 5-isoquinolinesulfonic acid was stirred in thionyl chloride and heated to reflux at a reaction temperature of 75°C-85°C to obtain 5 - Isoquinolinesulfonyl chloride hydrochloride;
[0025] (2) Dissolve 5-isoquinolinesulfonyl chloride hydrochloride in ice water, add dichloromethane solution, slowly add saturated aqueous sodium bicarbonate solution under stirring, adjust the pH value of the solution to neutral and then separate the liquids, and the water phase is dichloromethane After methane extraction once, the organic phases were combined, and the organic phases were washed with water and saturated brine in turn, dried over anhydrous magnesium sulfate, and suction filtered to obtain a dichloromethane solution of 5-isoquinolinesulfonyl chloride;
[0026] (3) Slowly add the dichloromethane soluti...
Embodiment 1
[0031] Example 1 Isoquinoline-5-sulfonyl chloride hydrochloride
[0032] 5-Isoquinolinesulfonic acid (20 g), thionyl chloride (200 mL), and DMF (10 g) were successively added into a 500 mL round-bottomed single-necked flask, and refluxed for 5 hours. The unreacted thionyl chloride was distilled off under reduced pressure to obtain a yellowish solid. 50 mL of dichloromethane was added thereto, and suction filtered. The filter cake was washed with dichloromethane and vacuum-dried to obtain a white solid, namely 5-isoquinolinesulfonyl chloride hydrochloride (22 g, 88%).
Embodiment 2
[0033] Example 2 5-isoquinolinesulfonyl chloride
[0034] Get the hydrochloride (19.4g, 73.4mmol) of 5-isoquinolinesulfonyl chloride obtained in Example 1 and put it into a 1L Erlenmeyer flask containing 300mL of ice-water mixture, then add 150mL of dichloromethane, and stir Neutralize the pH of the mixed liquid system to 7 with saturated aqueous sodium bicarbonate solution, separate and extract, keep the organic phase, extract the aqueous phase with dichloromethane (50mL) again, combine the organic phases, and dry the organic phase over anhydrous magnesium sulfate for half an hour After suction filtration, 200 ml of a dichloromethane solution of 5-isoquinolinesulfonyl chloride was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com