Preparation of piperaquini phosphatis
A technology of piperaquine phosphate and quinoline, which is applied in the field of preparation of raw materials, can solve the problems of large environmental pollution, high production cost, and low product yield, and achieve low environmental pollution, low production cost, and high product yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
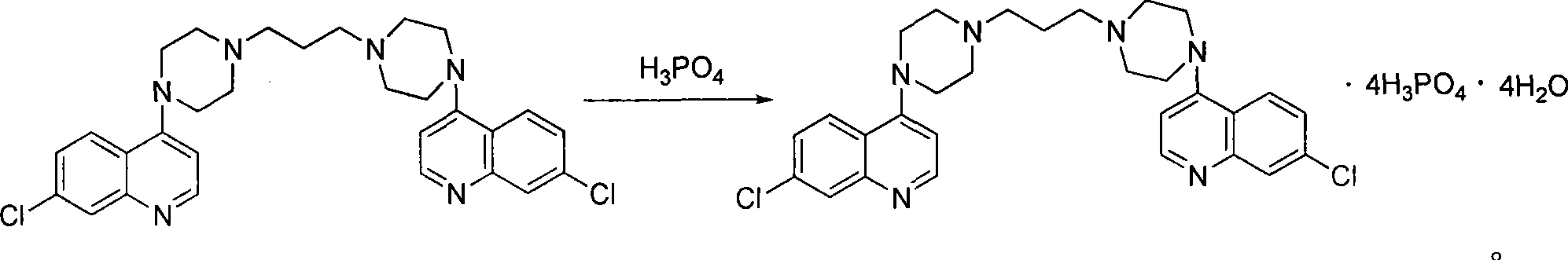
[0021] Embodiment 1, the preparation of piperaquine phosphate
[0022] Include the following steps:
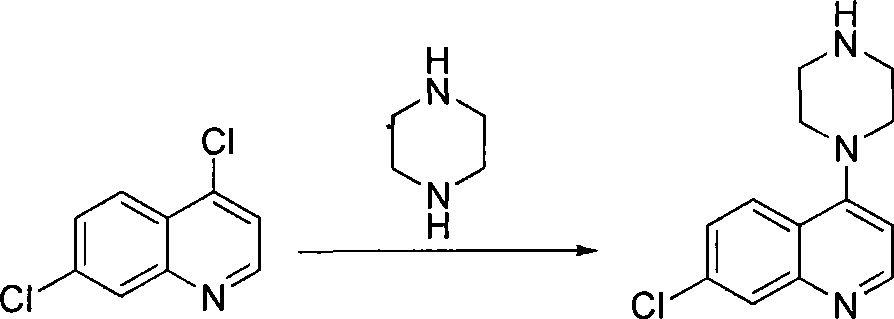
[0023] The preparation of a, 7-chloro-4-(1-piperazinyl) quinoline
[0024] According to the theoretical production amount of 7-chloro-4-(1-piperazinyl) quinoline 74.3g, calculate the amount of raw materials; in a 2000mL three-necked round-bottomed flask, add 4,7-dichloroquinoline 59.4g (0.3mol), Anhydrous piperazine 206.4g (2.4mol) and potassium carbonate 41.4g (0.3mol), then add water 600mL, reflux reaction 36 hours, monitor reaction progress with thin-layer chromatography (TLC) method, after the reaction is complete, the reaction solution Distilled under reduced pressure to 1 / 2 volume, extracted 3 times with 600 mL of ethyl acetate, collected and combined the ethyl acetate extracts, concentrated, and dried under vacuum at 55°C to obtain 7-chloro-4-(1-piperazinyl ) quinoline 72.8g, purity 98.5%, yield 98.0%;
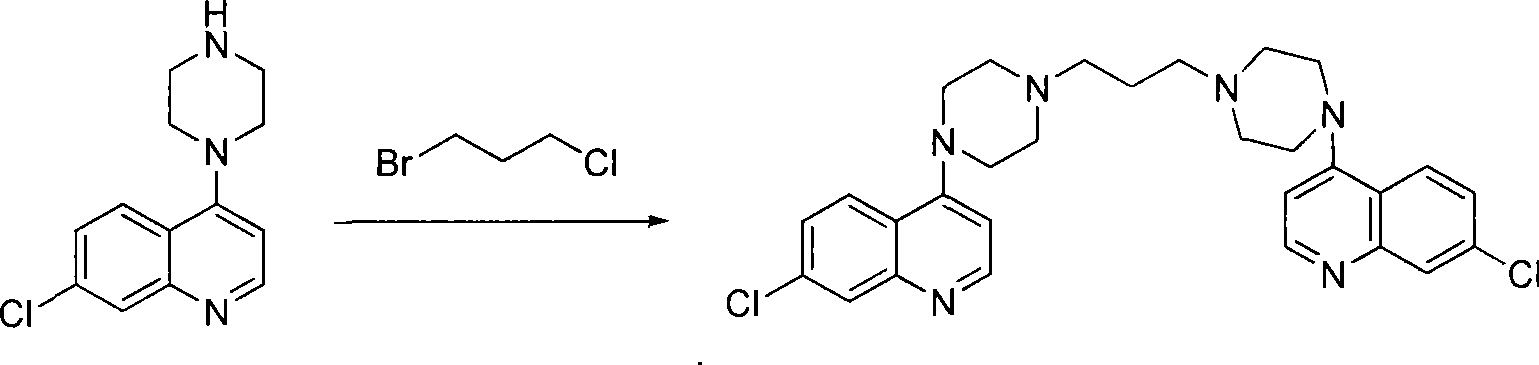
[0025] b, the preparation of piperaquine
[0026] Calculate th...
Embodiment 2
[0030] Embodiment 2, the preparation of piperaquine phosphate
[0031] Include the following steps:
[0032] The preparation of a, 7-chloro-4-(1-piperazinyl) quinoline
[0033] According to the theoretical production amount of 7-chloro-4-(1-piperazinyl) quinoline 74.3g, calculate the amount of raw materials; in a 2000mL three-necked round-bottomed flask, add 4,7-dichloroquinoline 59.4g (0.3mol), Anhydrous piperazine 258g (3mol) and salt of wormwood 41.4g (0.3mol), add methanol 600mL again, reflux reaction 36 hours, monitor the progress of reaction with TLC method, after the reaction is complete, the reaction solution is distilled off under reduced pressure to remove methanol, and then Add 300 mL of water, extract with 600 mL of dichloromethane for 3 times, collect and combine the dichloromethane extracts, concentrate, and dry under vacuum at 55°C to obtain 73.2 g of 7-chloro-4-(1-piperazinyl)quinoline, Purity 99.2%, yield 98.5%;
[0034] b, the preparation of piperaquine
...
Embodiment 3
[0039] Embodiment 3, the preparation of piperaquine phosphate
[0040] Include the following steps:
[0041] The preparation of a, 7-chloro-4-(1-piperazinyl) quinoline
[0042] According to the theoretical production amount of 7-chloro-4-(1-piperazinyl) quinoline 74.3g, calculate the amount of raw materials; in a 2000mL three-necked round-bottomed flask, add 4,7-dichloroquinoline 59.4g (0.3mol), Anhydrous piperazine 309.6g (2.6mol) and potassium carbonate 41.4g (0.3mol), then add propanol 600mL, reflux reaction for 36 hours, monitor the progress of the reaction with TLC method, after the reaction is complete, the reaction solution is distilled off under reduced pressure Propanol, then add 300mL of water, extract with 600mL of ethyl acetate three times, collect and combine the ethyl acetate extracts, concentrate, and dry under vacuum at 55°C to obtain 7-chloro-4-(1-piperazinyl)quinone 74.0g of morphine, with a purity of 98.4%, and a yield of 99.6%;
[0043] b, the preparation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com