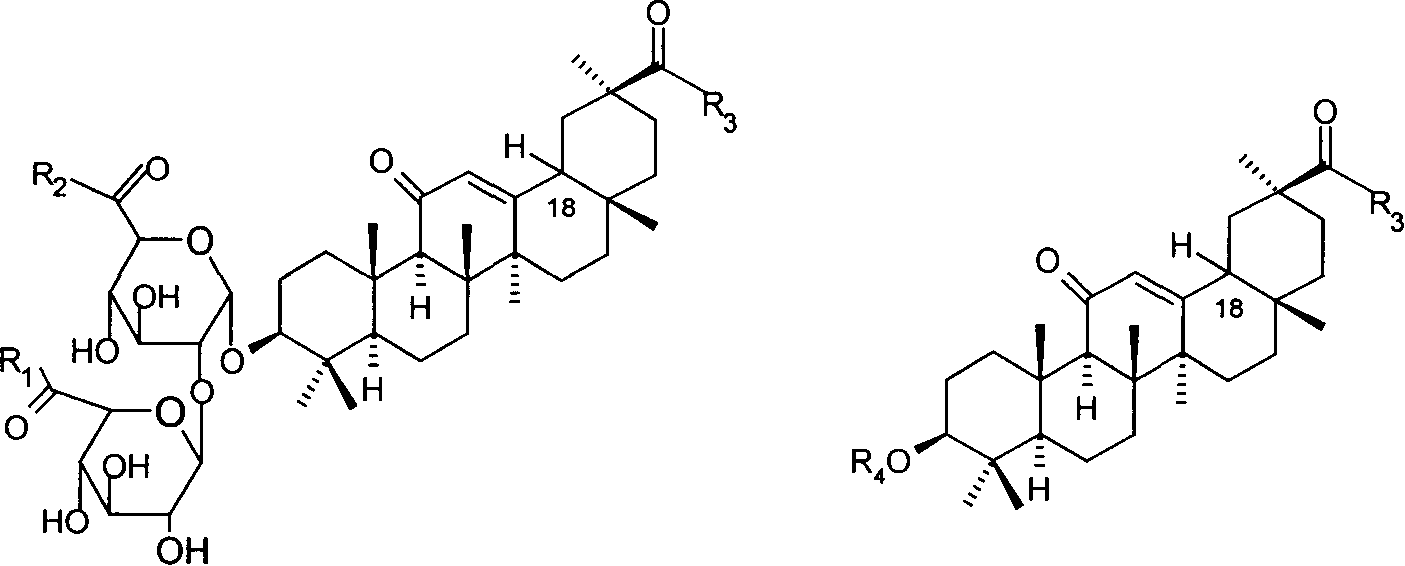
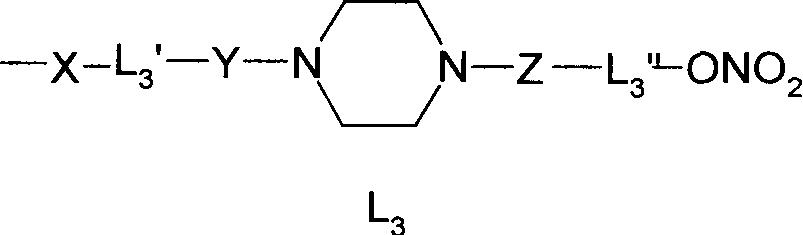
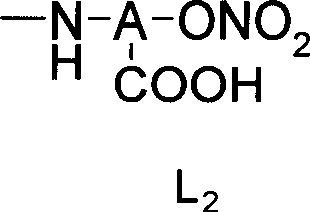Nitrate derivatives of glycyrrhetic acid and glycyrrhetinic acid and pharmaceutical use thereof
A kind of technology of glycyrrhetic acid, nitrate, applied in the application field of aspect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 18α glycyrrhizinyl-tris(2-nitrooxy-ethyl)-ester (II 1 ) Preparation 1a, 18α Glycyrrhizinyl-tris(2-bromo-ethyl)-ester
[0046] Suspend 1.78 g (2 mmol) of 18α trisodium glycyrrhizinate in 10 ml of DMF, add dropwise 1.5 g (8 mmol) of 1,2-dibromoethane, react at room temperature for 22 hours, filter, remove sodium bromide, and depressurize the filtrate Evaporate to dryness, separate the residue by silica gel column chromatography, elute with methanol / dichloromethane (1:1), collect the desired components, and evaporate to dryness under reduced pressure to obtain 1.86 g of 18α-glycyrrhizinyl-tri(2-bromo -ethyl)-ester. 1b, 18α glycyrrhizinyl-tris(2-nitrooxy-ethyl)-ester (II 1 ) preparation
[0047] Dissolve 1.86 g (1.63 mmol) of 18α glycyrrhizinyl-tris(2-bromo-ethyl)-ester in 10 ml of acetonitrile, 1.1 g (6.52 mmol) of AgNO 3 Dissolve in 2 ml of acetonitrile. AgNO 3 The acetonitrile solution was added dropwise to the solution of glycyrrhizinyl-tris(2-bromo-ethy...
Embodiment 2
[0048] Example 2 18α glycyrrhizinyl-tris(4-nitrooxy-butyl)-ester (II 2 ) Preparation of 2a, 18α Glycyrrhizinyl-tris(4-bromo-butyl)-ester
[0049] Suspend 1.78 g (2 mmol) of 18α trisodium glycyrrhizinate in 10 ml of DMF, add dropwise 1.73 g (8 mmol) of 1,4-dibromobutane, react at room temperature for 22 hours, filter, remove sodium bromide, and depressurize the filtrate Evaporate to dryness, separate the residue by silica gel column chromatography, elute with methanol / dichloromethane (1:1), collect the desired components, and evaporate to dryness under reduced pressure to obtain 1.37 g of 18α-glycyrrhizinyl-tri(4-bromo -butyl)-ester. 2b, 18α glycyrrhizinyl-tris(4-nitrooxy-butyl)-ester (II 2 ) preparation
[0050] Dissolve 1.37 g (1.21 mmol) of 18α glycyrrhizinyl-tris(4-bromo-butyl)-ester in 10 ml of acetonitrile, 0.82 g (4.84 mmol) of AgNO 3 Dissolve in 2 ml of acetonitrile. AgNO 3 The acetonitrile solution was added dropwise to the solution of glycyrrhizinyl-tri(4-bromo-...
Embodiment 3
[0051] Example 3 18α Glycyrrhizinyl-tris(3-nitrooxy-3-methyl-propyl)-ester (II 3 ) preparation
[0052] 3a, 18α Glycyrrhizinyl-tris(3-bromo-3-methyl-propyl)-ester
[0053] Suspend 1.78 g (2 mmol) of 18α trisodium glycyrrhizinate in 10 ml of DMF, add dropwise 1.73 g (8 mmol) of 1,3-dibromobutane, react at room temperature for 22 hours, filter, remove sodium bromide, and depressurize the filtrate Evaporate to dryness, separate the residue by silica gel column chromatography, elute with methanol / dichloromethane (1:1), collect the desired components, and evaporate to dryness under reduced pressure to obtain 1.20 g of 18α-glycyrrhizinyl-tri(3-bromo -3-Methyl-propyl)-ester.
[0054] 3b, 18α glycyrrhizinyl-tris(3-nitrooxy-3-methyl-propyl)-ester (II 3 ) preparation
[0055] Dissolve 1.20 g (1.06 mmol) of 18α glycyrrhizinyl-tris(3-bromo-3-methyl-propyl)-ester in 10 ml of acetonitrile, 0.72 g (4.24 mmol) of AgNO 3 Dissolve in 2 ml of acetonitrile. AgNO 3 The acetonitrile solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com