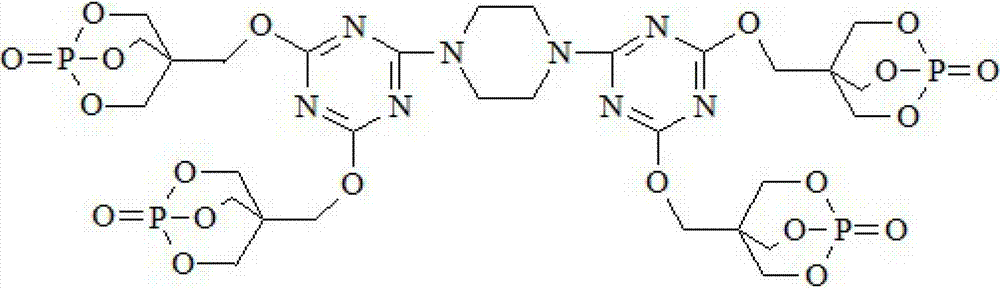
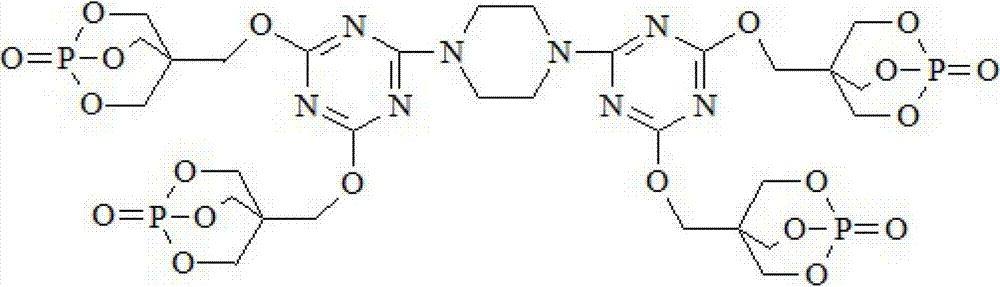
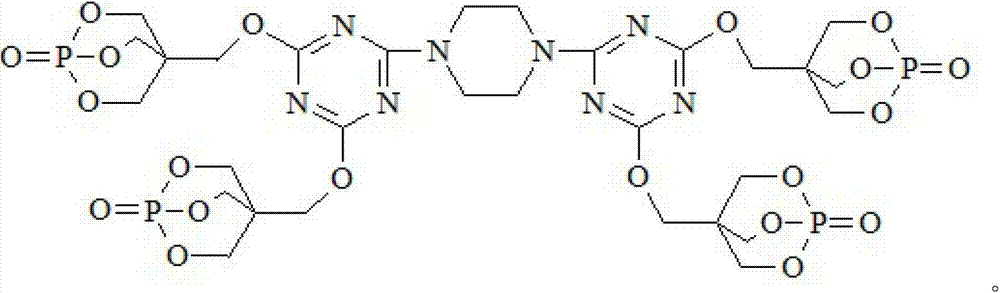
Triazine ring structure containing caged organic phosphate and preparation method thereof
A technology of caged phosphate ester and triazine ring, which is applied in the field of caged phosphate ester containing triazine ring structure and its preparation, can solve the problems of large amount of mixed intumescent flame retardants and poor water resistance, and achieve improved material The effect of deterioration of mechanical properties, improvement of compatibility, improvement of char formation and flame retardancy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Under the protection of high-purity nitrogen, add 92.2 g of cyanuric chloride and 800 mL of dioxane to a four-necked flask equipped with a stirrer, a thermometer, a reflux condenser, and a dropping funnel, and stir for 30 minutes to disperse the cyanuric chloride Evenly, add 21.5g of piperazine, control the temperature in the ice bath at 0~5°C, react for 4 hours, then add PEPA180.1g, heat up to 25°C, continue to stir and react for 6 hours, then add 151.8g of triethylamine The amine was heated to 70° C., and the reaction was continued for 2 hours. After filtering, washing and drying, the final product, a caged phosphate ester DTPTP containing a triazine ring structure, was obtained.
Embodiment 2
[0036] Under the protection of high-purity nitrogen, add 92.2 g of cyanuric chloride and 800 mL of N,N-dimethylformamide to a four-necked flask equipped with a stirrer, a thermometer, a reflux condenser, and a dropping funnel, and stir for 30 minutes to make The cyanuric chloride is evenly dispersed, then add 21.5g of piperazine, control the temperature in an ice bath at 0~5°C, and react for 6 hours, then add PEPA180.1g, heat up to 30°C, continue to stir and react for 8 hours, Add 177.1 g of triethylamine and raise the temperature to 80° C., continue the reaction for 4 hours, filter, wash and dry to obtain the final product, the caged phosphate DTPTP containing a triazine ring structure.
Embodiment 3
[0038] Under the protection of high-purity nitrogen, add 92.2 g of cyanuric chloride and 800 mL of N,N-dimethylacetamide to a four-necked flask equipped with a stirrer, a thermometer, a reflux condenser, and a dropping funnel, and stir for 30 minutes to make The cyanuric chloride is evenly dispersed, then add 21.5g of piperazine, control the temperature in an ice bath at 0~5°C, react for 4 hours, then add PEPA180.1g, heat up to 25°C, continue to stir and react for 6 hours, 118.7g of pyridine was added and the temperature was raised to 70°C. After the reaction was continued for 2 hours, the final product was filtered, washed and dried to obtain the caged phosphoric acid ester DTPTP containing a triazine ring structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com