High piperazine acetydrazide derivatives, and preparation and use thereof
A compound and prodrug technology, applied in the field of preparing the homopiperazine acetyl hydrazide derivatives, can solve problems that do not involve possible uses of novel compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
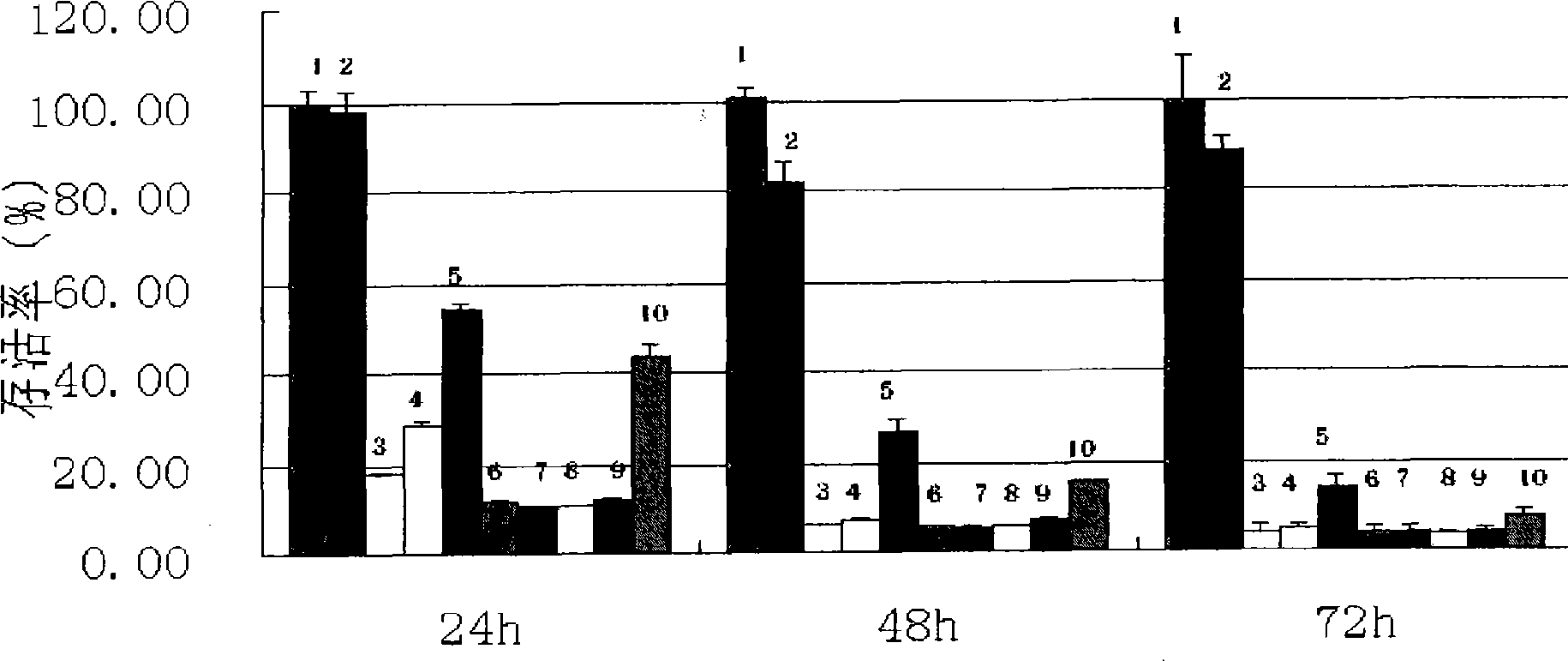
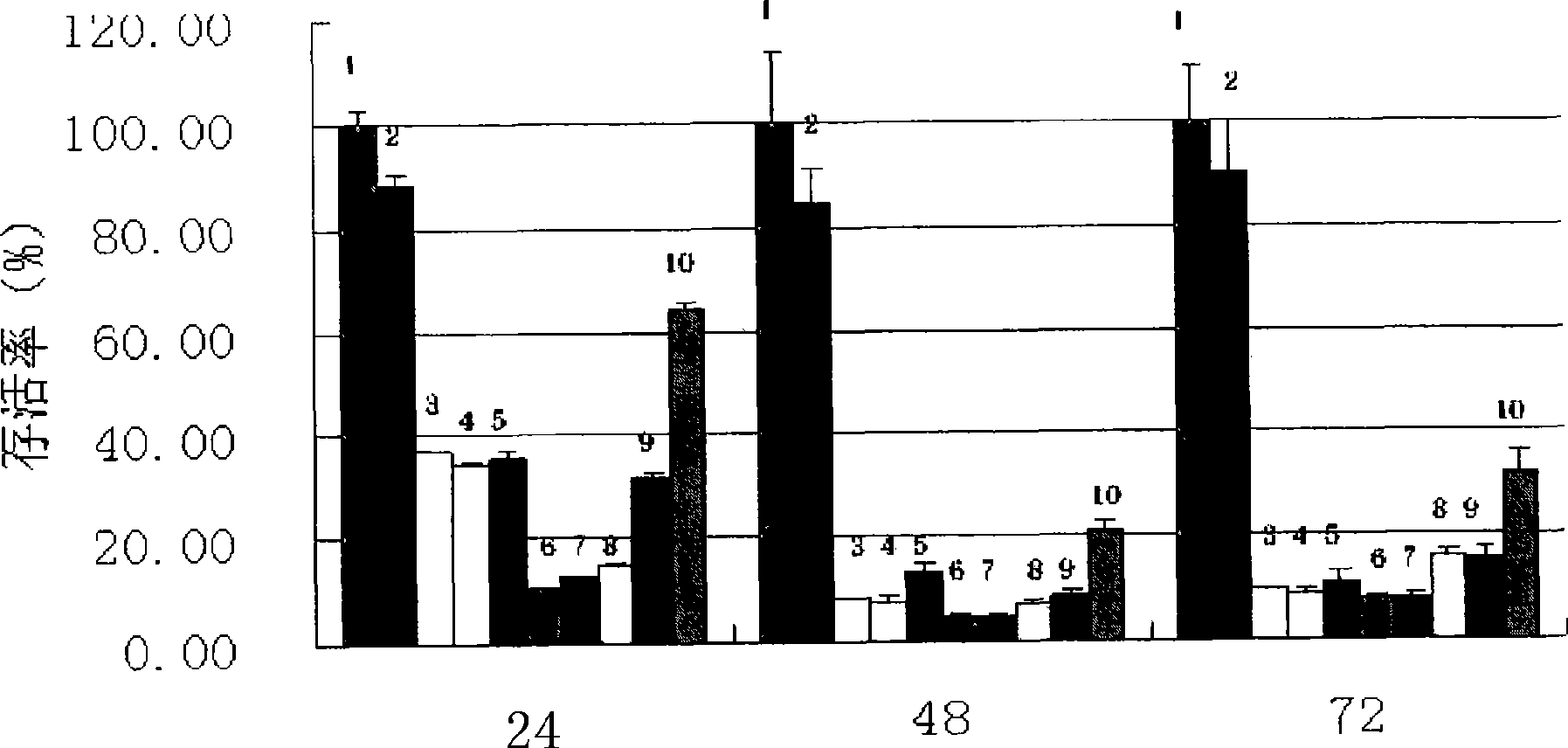
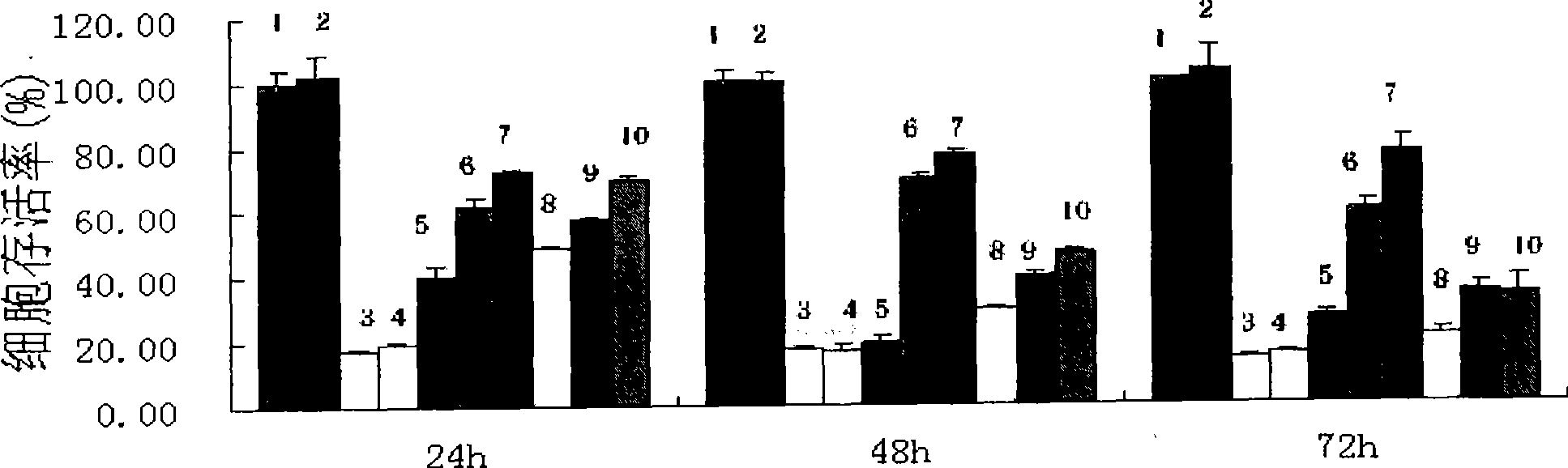
Examples
Embodiment 1
[0130] Example 1, (4-benzyl-[1,4]diazepan-1-yl)-acetyl (3-allyl-2-hydroxy Preparation of phenyl-methylene phenyl) hydrazine fumarate
[0131] The reaction flow diagram is as follows:
[0132]
[0133] step 1. Add 50 g (395 mmol) of benzyl chloride and 40 g (400 mmol) of homopiperazine into a 500 ml reaction flask, dissolve them in 300 ml of tetrahydrofuran, and heat to reflux for 5 hours. Cool, filter, and evaporate THF to a small amount. Add 300 ml of water, extract with ethyl acetate, use Na 2 SO 4 dry. Ethyl acetate was distilled off to obtain a crude product, which was separated on silica gel to obtain the pure product 1-benzylhomopiperazine with a yield of 95%.
[0134] Step 2. Under the condition of room temperature and electromagnetic stirring, add 9.5 g (50 mmol) of 1-benzyl homopiperazine and 7.35 g (60 mmol) of 2-chloroacetic ethyl ester and 100 ml of toluene solution to the reaction flask In, add 7.0 g (70 mmol) of triethylamine. After the reaction ...
Embodiment 2
[0140] Example 2, (4-phenyl-[1,4]diazepan-1-yl)-acetyl (2-hydroxyl-methylene Preparation of phenyl)hydrazine fumarate
[0141] The reaction flow diagram is as follows:
[0142]
[0143] step 1. Under the condition of room temperature and electromagnetic stirring, add 8.6 g (50 mmol) of 1-phenylhomopiperazine and 7.35 g (60 mmol) of 2-chloroacetate ethyl ester and 100 ml of toluene solution to the reaction flask In, add 7.0 g (70 mmol) of triethylamine. After the reaction solution was heated to reflux for 8 hours, the reaction was stopped, and the solvent was distilled off. The product was dissolved in 100 ml of ethyl acetate and washed twice with 20 ml of water each. The organic phase was dried over magnesium sulfate, the solvent was evaporated, and the obtained product was separated by column chromatography to obtain 12.4 g of liquid ethyl 4-phenylhomopiperazine-1-acetate with a yield of 92%.
[0144] Proton spectrum (400MHz, CDCl 3 ): 7.18-7.30(m, 5H); 4.08-4.13...
Embodiment 3
[0148] Example 3, (4-phenylethyl-[1,4]diazepan-1-yl)-acetyl (3,5-diallyl- Preparation of 2-Hydroxy-Methylenephenyl)hydrazine Fumarate
[0149] The reaction flow diagram is as follows:
[0150]
[0151]
[0152] step 1. Add 50 g (355 mmol) of 2-chloroethylbenzene and 40 g (400 mmol) of homopiperazine into a 500 ml reaction flask, dissolve them in 300 ml of tetrahydrofuran, and heat to reflux for 5 hours. Cool, filter, and evaporate THF to a small amount. Add 300 ml of water, extract with ethyl acetate, use Na 2 SO 4 dry. Ethyl acetate was distilled off to obtain a crude product, which was separated on silica gel to obtain the pure product 1-phenethylhomopiperazine with a yield of 80%.
[0153] Step 2. Under the condition of room temperature and electromagnetic stirring, react to 1-phenethyl homopiperazine and 7.35 grams (60 mmol) of 1-phenylethyl homopiperazine and 100 milliliters of toluene solution equipped with 10.2 grams (50 mmol) In the bottle, add 7.0 g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com