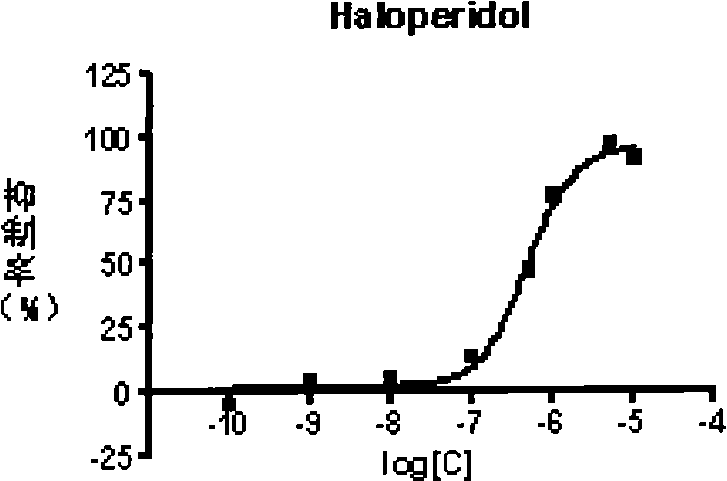
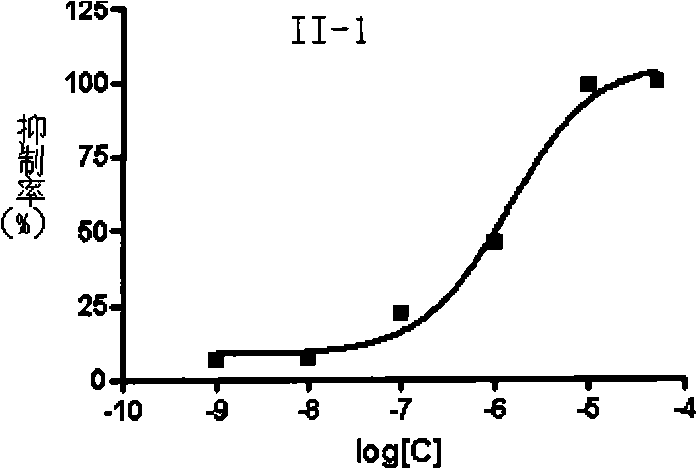
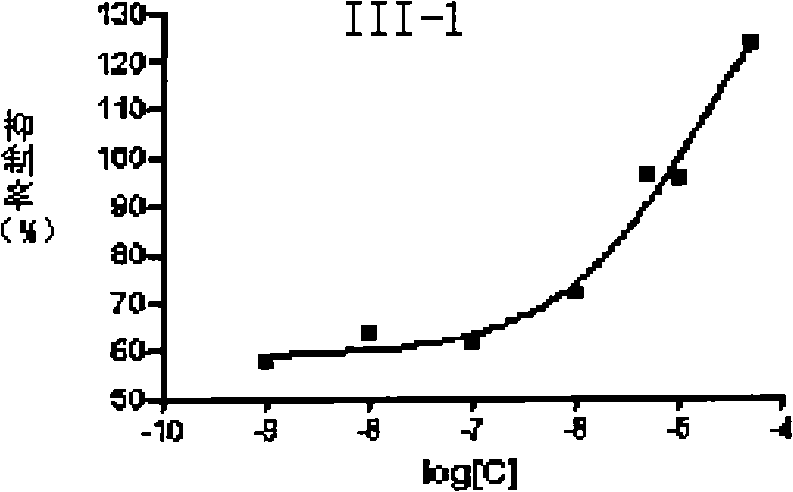Aralkyl piperidine (piperazidine) derivate and use thereof in mental disease treatment
A technology of aralkylpiperidine and derivatives is applied in the application field of preparing medicines for treating mental and neurological diseases, and can solve the problems of obvious symptoms, elevated prolactin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] I-17-[4-(4-(6-chloro-benzoisoxazole)-1-piperazinyl)-n-butoxy]-3,4-dihydro-2(1H)-quinolinone Hydrochloride
[0117] a) Preparation of 4-chloro-methyl salicylate
[0118] Slowly drop 5ml of concentrated sulfuric acid into 100ml of methanol, add 4-chloro-salicylic acid powder (17.20g, 0.1mol) after the solution is cooled, reflux for 24h, cool down, a large amount of precipitate precipitates, filter out, wash with a small amount of methanol, and Recrystallized from absolute ethanol to obtain 15.60 g of methyl 4-chloro-salicylate, with a yield of 83%.
[0119] b) Preparation of 4-chloro-N,2-dihydroxy-benzamide
[0120] Put (5.25g, 75mmol) hydroxylamine chloride solid in an eggplant-shaped bottle, add a small amount of water dropwise under ice bath to dissolve it, then add 15ml of 50% NaOH solution dropwise, stir for 5 minutes, and add 4 - Chloro-methyl salicylate (9.3g, 50mmol) in dioxane solution 50ml, react at room temperature for 24h after the dropwise addition, a redd...
Embodiment 2
[0135] I-27-[4-(4-(5-chloro-benzoisoxazole)-1-piperazinyl)-n-butoxy]-3,4-dihydro-2(1H)-quinolinone Hydrochloride
[0136] With 5-chloro-salicylic acid salicylic acid as the starting material, the target compound was synthesized according to the method of preparation I-1
[0137] Elemental Analysis: C 24 h 27 ClN 4 o 3 • 2HCl (theoretical %: C 63.36, H 5.98, N 12.31; found % C 62.72, H 5.92, N 12.24).
[0138] 1 HNMR (DMSO-d 6 ): δ10.03 (s, 1H, CONH), 7.99-6.46, (6H, aromatic ring-H), 4.10-4.15 (2H, piperazine-H), 3.99 (t, J=6.4HZ, 2H, O -CH2,) 3.50-3.70 (2H, piperazine-H), 3.22-3.57 (m, 6H), 2.75 (t, J=8HZ, 2H), 2.45 (t, J=8HZ, 2H) 1.71-1.98 ( m, 4H)
[0139] MS: m / z 454
Embodiment 3
[0141] I-37-[4-(4-(Benzisoxazole)-1-piperazinyl)-n-butoxy]-3,4-dihydro-2(1H)-quinolinone hydrochloride
[0142] Using salicylic acid as the starting material, the target compound was synthesized according to the method of preparation I-1.
[0143] Elemental Analysis: C 24 h 28 N 4 o 3 • 2HCl (theoretical %: C 68.55, H 6.71, N 13.32; found % C 68.00, H 6.65, N 13.21).
[0144] 1 HNMR (DMSO-d 6 ): δ10.00 (s, 1H, CONH), 7.96-6.48, (7H, aromatic ring-H), 4.05-4.10 (2H, piperazine-H), 3.93 (t, J=6.4HZ, 2H, O -CH2,) 3.59-3.62 (2H, piperazine-H), 3.20-3.50 (m, 6H), 2.78 (t, J=8HZ, 2H) 2.41 (t, J=8HZ, 2H) 1.73-1.93 (m , 4H)
[0145] MS: m / z 420
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com