Process for synthesizing imatinib
A synthesis method and imatinib technology, applied in the field of pharmaceutical compound synthesis, can solve the problems of difficulty in purification, instability, long reaction time, etc., and achieve the effects of being beneficial to industrial production, mild reaction conditions, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
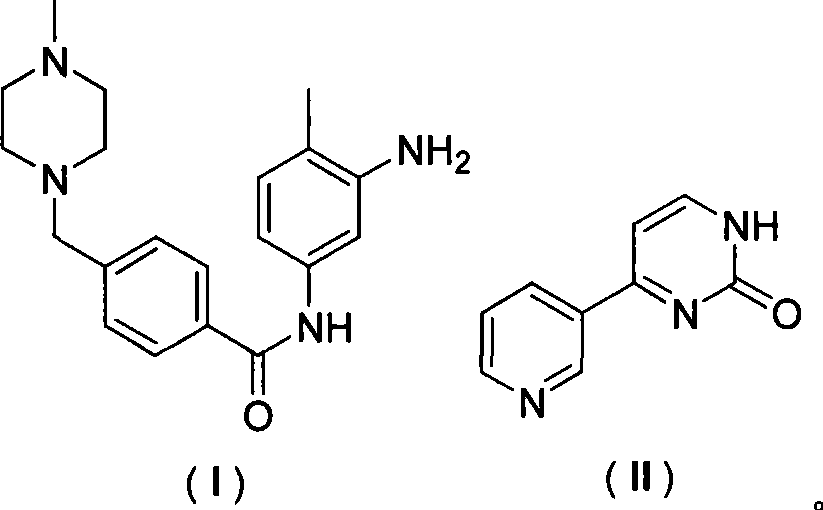
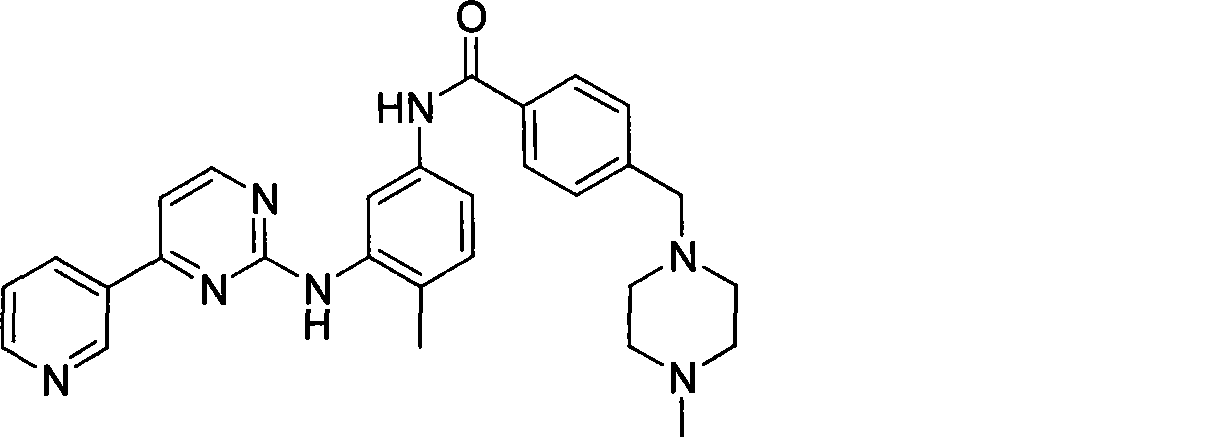
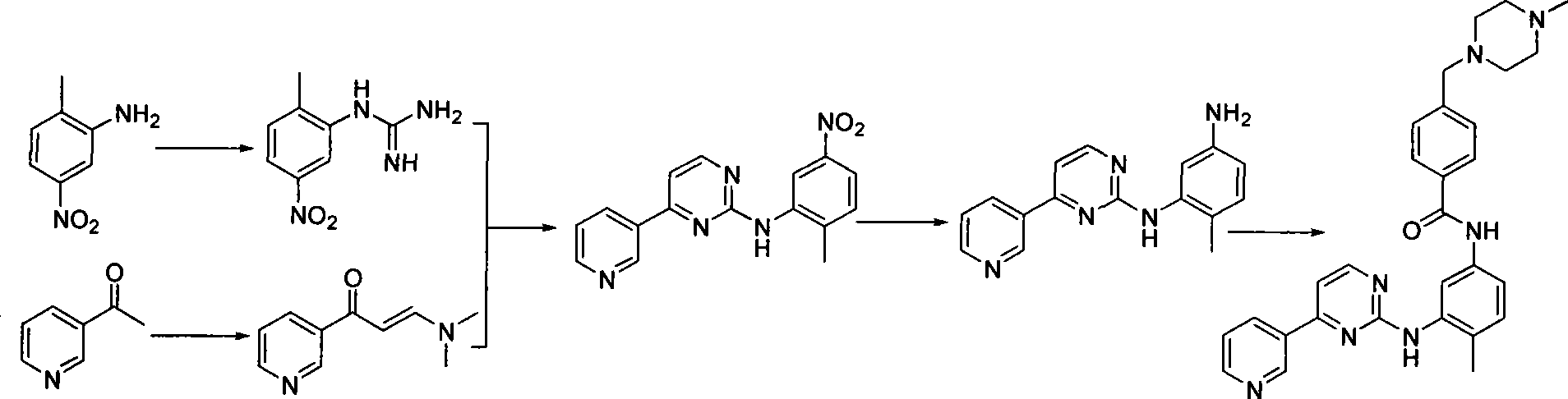
[0035] In a 500ml dry four-neck flask, add 250ml of tetrahydrofuran, 33.8g of N-(4-methyl-3-aminophenyl)-4-(4-methyl-piperazinyl-1-methyl)-benzamide and 17.3 g of 4-methyl-(3-pyridyl)-2-pyrimidinone, stirring and dissolving, cooling to 0 degrees, adding (benzotriazole-1-oxyl)-tris(dimethylamino)phosphonium hexa Fluorophosphate (BOP) 50g and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) 16g. Slowly raise the temperature to 25° C. and react overnight. After detecting the completion of the reaction, the tetrahydrofuran was concentrated and the obtained solid was washed with water and dried to obtain 45 g of imatinib, with a yield of 91.0%.
[0036] The spectral data are as follows:
[0037] 1 H NMR (500M, DMSO) δ: 10.2(s, 1H), 9.30(s, 1H), 8.99(s, 1H), 8.72(d, J=4.0Hz, 1H), 8.57(s, 1H), 8.53 (s, 1H), 8.11(s, 1H), 8.00(s, 1H), 7.98(s, 1H), 7.58-7.51(m, 4H), 7.44(d, J=4.3Hz, 1H), 7.22( d, J=8.1Hz, 1H), 3.70(s, 2H), 3.50-3.25(m, 2H), 3.20-2.90(m, 4H), 2.81(s, 3H), 2.40(s, 3H), 2.24 ...
Embodiment 2
[0040] In a 500ml dry four-necked flask, add 250ml of acetonitrile, 33.8g of N-(4-methyl-3-aminophenyl)-4-(4-methyl-piperazinyl-1-methyl)-benzamide and 17.3 g of 4-methyl-(3-pyridyl)-2-pyrimidinone, stirring and dissolving, cooling to 0 degrees, adding (benzotriazole-1-oxyl)-tris(dimethylamino)phosphonium hexa Fluorophosphate (BOP) 50g and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) 16g. Slowly raise the temperature to 25° C. to react overnight. After detecting the completion of the reaction, concentrate the acetonitrile, wash the obtained solid with water, and dry to obtain 44 g of imatinib, with a yield of 89.0%. Spectral data ditto.
Embodiment 3
[0042] In a 5000ml dry four-necked bottle, add toluene 2500ml, N-(4-methyl-3-aminophenyl)-4-(4-methyl-piperazinyl-1-methyl)-benzamide 338g and 173 g of 4-methyl-(3-pyridyl)-2-pyrimidinone, 500 g of tripyrrolidinylphosphonium bromide hexafluorophosphate (PyBrOP) and 160 g of triethylamine were added under stirring. After reacting overnight, the toluene was concentrated after detecting the completion of the reaction, and the obtained solid was washed with water and dried to obtain 445 g of imatinib, with a yield of 90.0%. Spectral data ditto.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com