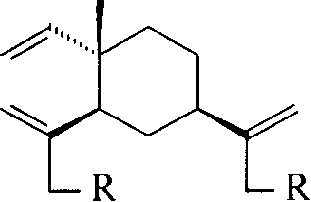
Beta-elemene derivatives containing nitrogen and their preparation method and use
A derivative, the technology of elemene, which is applied in the field of application of nitrogen-containing derivatives of β-elemene, can solve the problems of weak anticancer effect, insignificant curative effect, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
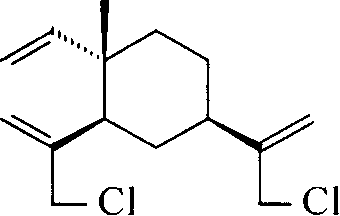
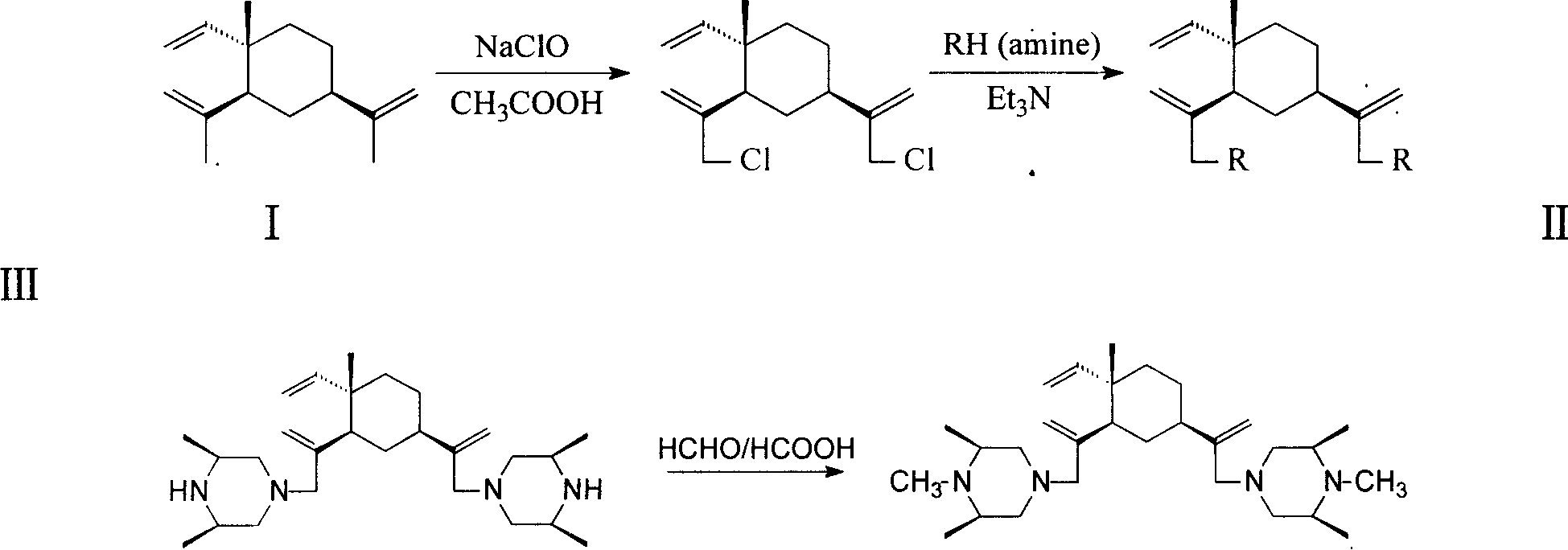
[0027] The preparation of embodiment 1 dichloro-beta-elemene intermediate (II)
[0028] Add 26.0 g (0.127 mol) of β-elemene and 38 mL (0.67 mol) of glacial acetic acid into a three-necked bottle equipped with a mechanical stirrer, cool it to about 5°C with an ice-water bath, and add 198 mL of sodium hypochlorite solution dropwise under stirring ( 1.41mol / L, 0.275mol), the dropwise addition was completed in about 4 hours, and the reaction was continued for 1 hour. The reaction solution was transferred to a 1L separatory funnel, extracted twice with 50mL petroleum ether (60-90°C), the organic phases were combined, washed with water until neutral, and dried over anhydrous sodium sulfate. Petroleum ether was distilled off to obtain 30.5 g of a brown oily substance. GC showed that the product contained 15.0% monochloro-elemene and 54.6% dichloro-elemene. It was separated by silica gel column chromatography and eluted with petroleum ether.
Embodiment 2
[0029] General method for the preparation of embodiment 2 double-substituted β-elemene nitrogen-containing derivatives
[0030] Dissolve 16mmol of amine, 4mmol of dichloro-β-elemene and 16mmo of triethylamine in 10mL of N,N-dimethylformamide and reflux for 8-20h. The formed triethylamine hydrochloride needle crystals were filtered out, and 10 mL of water was added to the filtrate, extracted 4 times with petroleum ether-diethyl ether, the extract was dried and concentrated, and separated by silica gel column chromatography to obtain the product.
Embodiment 3
[0031] Example 3 Synthesis
[0032] Using 3,5-dimethylpiperazine as a raw material, it was prepared according to the general method for preparing nitrogen-containing derivatives of disubstituted β-elemene in Example 2. Add maleic acid acetone solution dropwise to form maleate to obtain white crystals, mp192.0-194.5°C. MS (m / z): 429 (M+1); 1 H-NMR (CDCl 3 )δ (ppm): 1.00 (15H, m), 1.40-1.65 (8H, m), 2.09 (4H, m), 2.60-2.98 (12H, m, ), 4.78-5.03 (6H, m), 5.76- 5.82 (1H,dd)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com