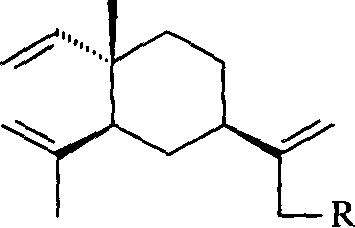
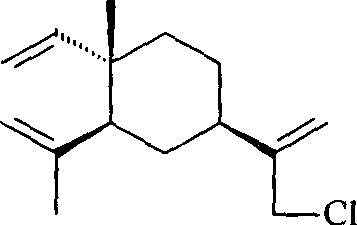
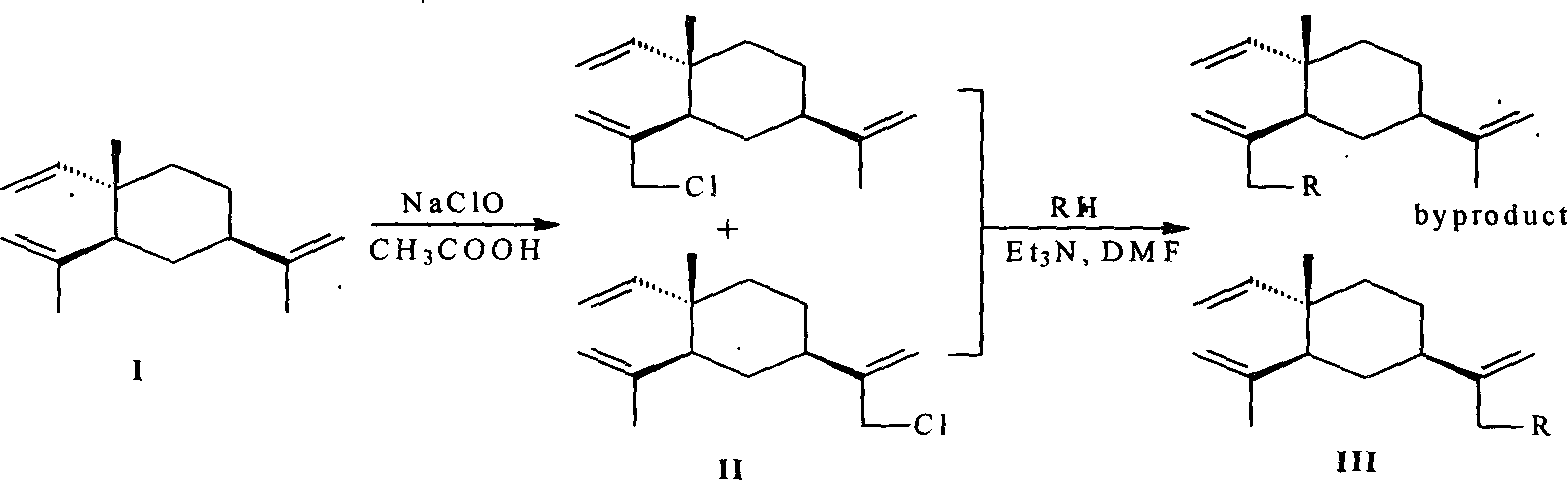
Beta-element nitrogenous derivative, and its preparing method and use
A derivative, the technology of elemene, which is applied in the application field of nitrogen-containing derivatives of β-elemene, can solve the problems of weak anticancer effect, poor stability, and ineffective curative effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Chlorinated β-elemene intermediate and preparation of
[0026] Add 51.0g (0.25mol) of β-elemene and 35mL (0.61mol) of glacial acetic acid into a three-necked flask equipped with a mechanical stirrer, cool to about 5°C with an ice-water bath, and add 180mL (1.41mol) dropwise under stirring. / L, 0.254mol) sodium hypochlorite solution, the dropwise addition is completed in about 4 hours, and the reaction is continued for 1 hour. Then the reaction solution was transferred to a separatory funnel, extracted twice with 50 mL of petroleum ether (60-90° C.), the organic phases were combined, washed with water until neutral, dried over anhydrous sodium sulfate, and concentrated to obtain 52.5 g of light yellow-green oil. GC showed 41.4% of unreacted elemene and 44.5% of monochloro-elemene, a mixture of 13-chloro-β-elemene and 14-chloro-β-elemene. It was separated by silica gel column chromatography and eluted with petroleum ether.
Embodiment 2
[0027] General method for the preparation of embodiment 2 β-elemene nitrogen-containing derivatives
[0028] Dissolve 10mmol of amine, 5mmol of monochlorinated β-elemene and 10mmol of triethylamine in 5mL of N,N-dimethylformamide and reflux for 8-20h. The resulting triethylamine hydrochloride needle crystals were filtered out, and the filtrate was added with 10 mL of water, extracted 4 times with petroleum ether-diethyl ether, the extract was dried and concentrated, and separated by thin-layer preparation with silica gel.
Embodiment 3
[0029] Example 3 Synthesis
[0030] Using 2-methylpiperazine as a raw material, it was prepared according to the general method for the preparation of nitrogen-containing derivatives of β-elemene in Example 2. Add maleic acid acetone solution dropwise to form maleate to obtain white crystals, mp123.0-125.9°C. MS(m / z): 302[M + ]; 1 H-NMR (CDCl 3 )δ (ppm): 0.99 (3H, s), 1.03 (3H, d), 1.45-1.64 (6H, m), 1.71 (3H, s), 1.91-2.00 (4H, m), 2.72-2.95 (7H , m), 4.59-4.93 (6H, m), 5.81 (1H, dd)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com