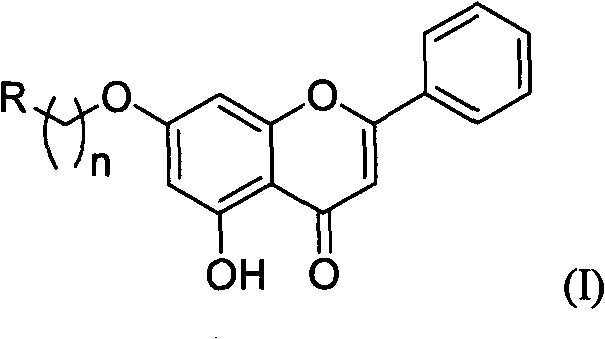
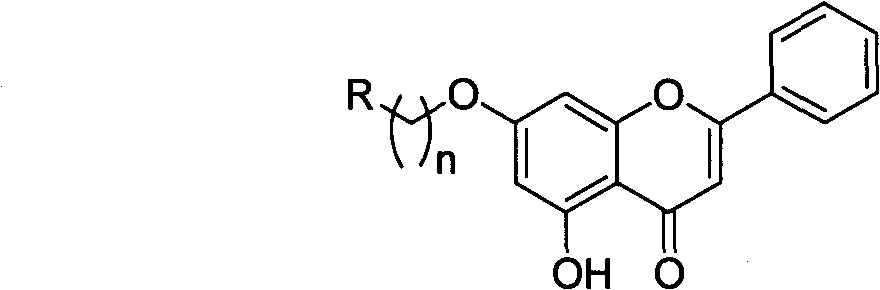
Chrysin nitrogen-containing derivative as well as preparation method and purpose thereof
A kind of derivative, the technology of chrysin, which is applied in the application field of the nitrogen-containing derivative of chrysin, can solve the problems of poor water-solubility and fat-solubility, reduced activity, difficult to reach the action site and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
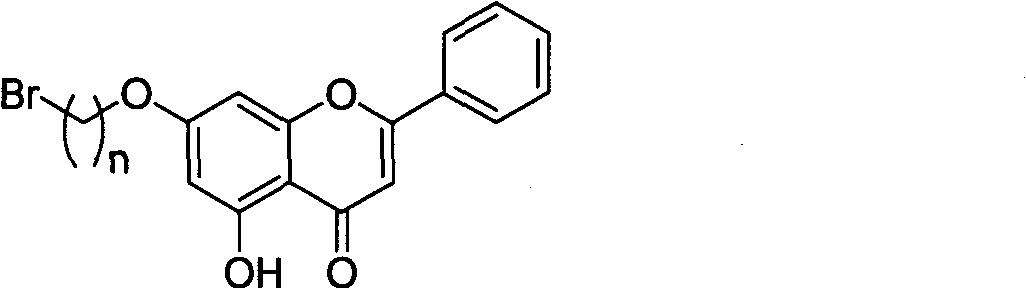
[0024] Embodiment 1 bromochrysin intermediate preparation of
[0025] Chrysin (500mg, 1.96mmol), 1,2-dibromoethane (3mL, 0.035mol), K 2 CO 3 (544mg, 3.93mmol), acetone 35mL, heated to reflux for 24h. The solvent was evaporated under reduced pressure and 50 mL of water was added to the system, and the K 2 CO 3 Dissolved and filtered to obtain a light yellow crude product, which was recrystallized from absolute ethanol to obtain a light yellow solid powder. FAB-MS, m / z: 362 (M+1); 1 H NMR (CDCl 3 , 500MHz) δ: 3.84~3.86 (2H, t, J=10.5Hz, OCH 2 C H 2 ), 4.46~4.48 (2H, t, J=10.5Hz, OCH 2 ), 6.43(1H, s, H-6), 6.88(1H, s, H-8), 7.06(1H, s, H-3), 7.58~7.65(3H, m, H-4', 5' , 6'), 8.10~8.12(2H, m, H-2', 3'); 13 C NMR (CDCl 3 , 500MHz) δ: 30.7 (OCH 2 C h 2 ), 68.4 (CH 2 ), 93.4(C-8), 98.5(C-6), 105.1(C-3), 105.3(C-10), 126.4(C-2', 6'), 129.0(C-3', 5' ), 130.5(C-4'), 132.1(C-1'), 157.2(C-9), 161.2(C-5), 163.5(C-2), 163.8(C-7), 182.0(C- 4)
Embodiment 2
[0026] Embodiment 2 bromochrysin intermediate preparation of
[0027] Chrysin (500mg, 1.96mmol), 1,6-dibromohexane (0.598mL, 3.92mmol), K 2 CO 3 (544mg, 3.93mmol), acetone 35mL, heated to reflux for 24h. The solvent was evaporated under reduced pressure and 50 mL of water was added to the system, and the K 2 CO 3 Dissolved and filtered to obtain a light yellow crude product, which was recrystallized from absolute ethanol to obtain a light yellow solid powder. FAB-MS, m / z: 417 (M+1); 1 H NMR (CDCl 3 , 500MHz) δ: 1.38~1.42 (2H, m, H-4"), 1.44~1.58 (2H, m, H-3"), 1.78~1.82 (2H, m, H-5"), 2.22~2.38 (4H, m, H-2", 6"), 4.12 (2H, t, J=10.8Hz, H-1"), 6.43 (1H, s, H-6), 6.89 (1H, s, H- 8), 7.05(1H, s, H-3), 7.57~7.63(3H, m, H-4', 5', 6'), 8.12~8.14(2H, m, H-2', 3') ; 13 C NMR (CDCl 3, 500MHz) δ: 25.2(C-3”), 28.3(C-4”), 29.8(C-2”), 32.5(C-5”), 33.6(C-6”), 68.8(C-1 ”), 93.5 (C-8), 98.4 (C-6), 105.1 (C-3), 105.3 (C-10), 126.4 (C-2’, 6’), 129.0 (C-3’, 5 '), 130.5(C-4'), 132.1...
Embodiment 3
[0028] Embodiment 3 bromochrysin intermediate preparation of
[0029] Chrysin (500mg, 1.96mmol), 1,20-dibromoeicosane (1.03g, 2.35mol), K 2 CO 3 (544mg, 3.93mmol), acetone 45mL, heated to reflux for 24h. The solvent was evaporated under reduced pressure and 50 mL of water was added to the system, and the K 2 CO 3 Dissolved and filtered to obtain a light yellow crude product, which was recrystallized from absolute ethanol to obtain a light yellow solid powder. FAB-MS, m / z: 613 (M+1); 1 H NMR (CDCl 3 , 500MHz) δ: 1.28~1.32 (32H, m, H-3”~H-18”), 1.71~1.76 (4H, m, H-2”, 19”), 3.28~3.31 (2H, t, J =9.6Hz, H-20"), 3.93~3.95 (2H, t, J=9.7Hz, H-1"), 6.44 (1H, s, H-6), 6.88 (1H, s, H-8) , 7.06(1H, s, H-3), 7.58~7.66(3H, m, H-4', 5', 6'), 8.09~8.11(2H, m, H-2', 3'); 13 C NMR (CDCl 3 , 500MHz) δ: 26.2 (C-2”), 28.2 (C-18”), 28.9 (C-17”), 29.6 (C-3”~C-16”), 32.8 (C-19”), 33.9(C-20"), 68.9(C-1"), 93.4(C-8), 98.6(C-6), 105.1(C-3), 105.3(C-10), 126.4(C-2' , 6'), 129.0(C-3', 5'), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com