Benzenesulfonyl piperazine compounds or benzoyl piperazine compounds, preparation methods and uses thereof
A kind of compound, the technology of piperazine, be used in the pharmaceutical composition that comprises described compound, prepare described compound field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
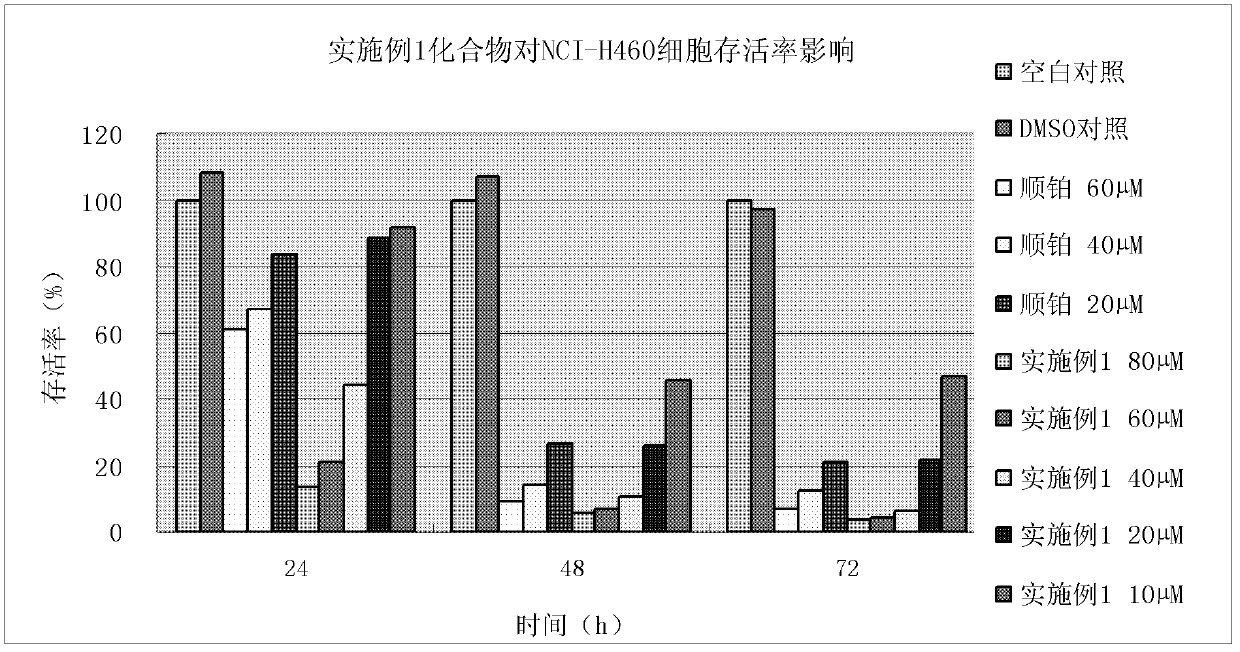
Embodiment 1
[0139] Example 1, 2-(4-(4-(4-methylpiperazin-1-yl)benzenesulfonyl)piperazin-1-yl)-acetyl (3-allyl-2-hydroxyl-methylene The preparation of phenyl) hydrazine
[0140] The reaction process is as follows:
[0141]
[0142] Step 1. Add piperazine (25.8 g, 0.3 mol) and DCM (50 ml) into the reaction flask under a water bath at room temperature, and stir to gradually dissolve the solid to form a suspension. Then slowly add DCM (50ml) of p-bromobenzenesulfonyl chloride (25.5g, 0.1mol) dropwise, stir for 30min after completion of the dropwise addition, TLC monitoring reaction ends, then add tap water (50ml) to wash once, separate the DCM layer, water Layer was extracted with DCM (50ml), separated, and the DCM layers were combined, and the DCM was removed by rotary evaporation, and a solid was precipitated. The solid was basically dissolved with methanol (30ml), and then tap water (100ml) was slowly added dropwise. A large amount of solids were precipitated, filtered, and filtered. ...
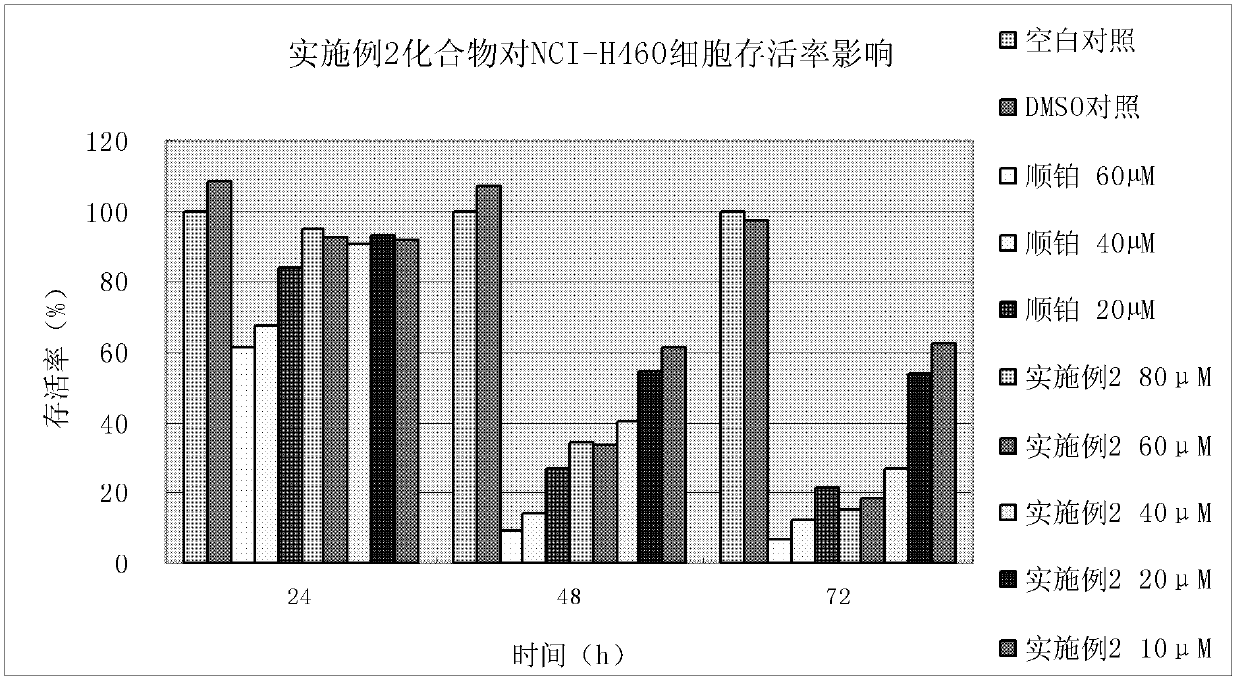
Embodiment 2
[0147] Embodiment 2, the preparation of 2-(4-(4-morpholinylbenzenesulfonyl)piperazin-1-yl)-acetyl(3-allyl-2-hydroxyl-methylenephenyl)hydrazine
[0148] The reaction process is as follows:
[0149]
[0150] Step 1. Add (3.05g, 10mmol) compound 1 (see Example 1 for the preparation method), N-methylpyrrolidone (10ml) and morpholine (2.61g, 30mol) into the reaction flask, stir at room temperature to dissolve all the solids, Then put the reaction bottle into an oil bath, seal it with a balloon, and raise the temperature to 170°C for a constant temperature reaction until the raw materials disappear. After 15 hours of reaction, the reaction was completed, cooled to room temperature, then poured into a 50ml beaker, added DCM (10ml), stirred evenly, then added tap water (100ml), extracted, separated the DCM layer, and used tap water (4*20ml) After washing four times, the DCM layer was separated, dried over anhydrous magnesium sulfate (2 g), filtered, and the filter cake was washed ...
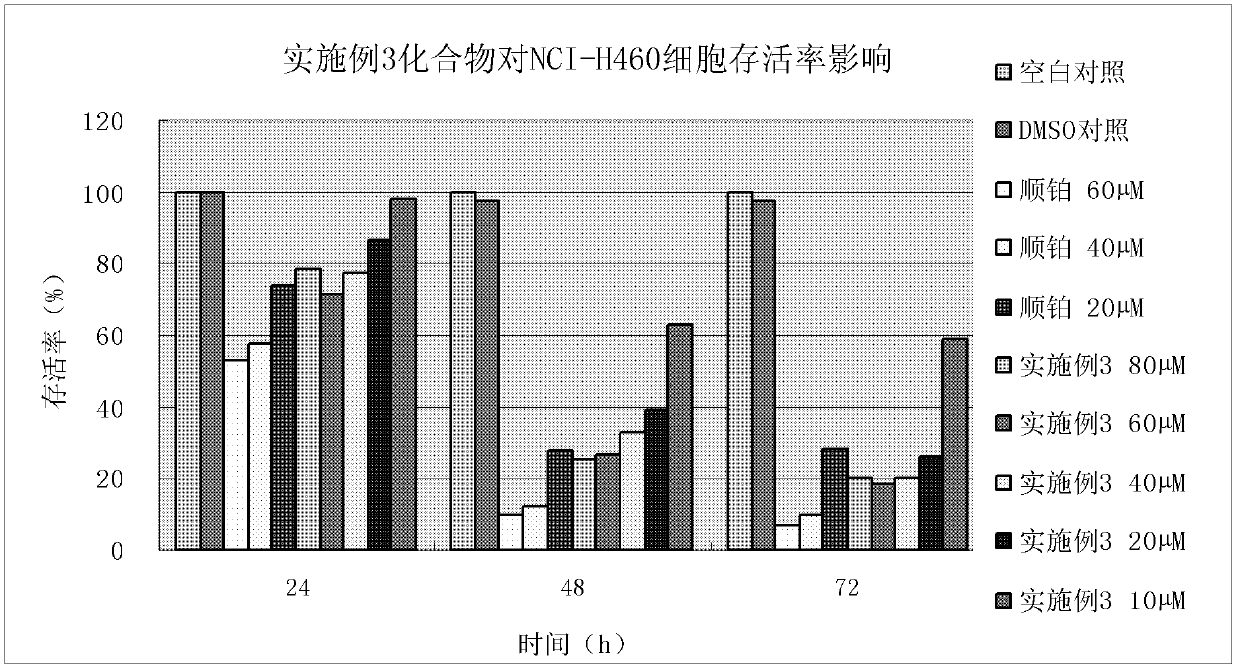
Embodiment 3
[0154] Example 3, Preparation of 2-(4-(p-toluenesulfonyl)piperazin-1-yl)-acetyl(3-allyl-2-hydroxyl-methylenephenyl)hydrazine
[0155] The reaction process is as follows:
[0156]
[0157] Step 1. Add piperazine (25.8 g, 0.3 mol) and DCM (50 ml) into the reaction flask under a water bath at room temperature, and stir to gradually dissolve the solid to form a suspension. Then slowly add DCM (50ml) of p-toluenesulfonyl chloride (25.5g, 0.1mol) dropwise, stir for 30min after completion of the dropwise addition, TLC monitoring reaction ends, then add tap water (50ml) to wash once, separate DCM layer, water layer Extract with DCM (50ml), separate, combine the DCM layers, remove the DCM by rotary evaporation, and precipitate a solid, which is basically dissolved with methanol (30ml), and then slowly add tap water (100ml) dropwise, a large amount of solids precipitate, filter, filter cake Rinse with water. After infrared drying, 24 g of white solid was obtained; yield: 80%. Mass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com