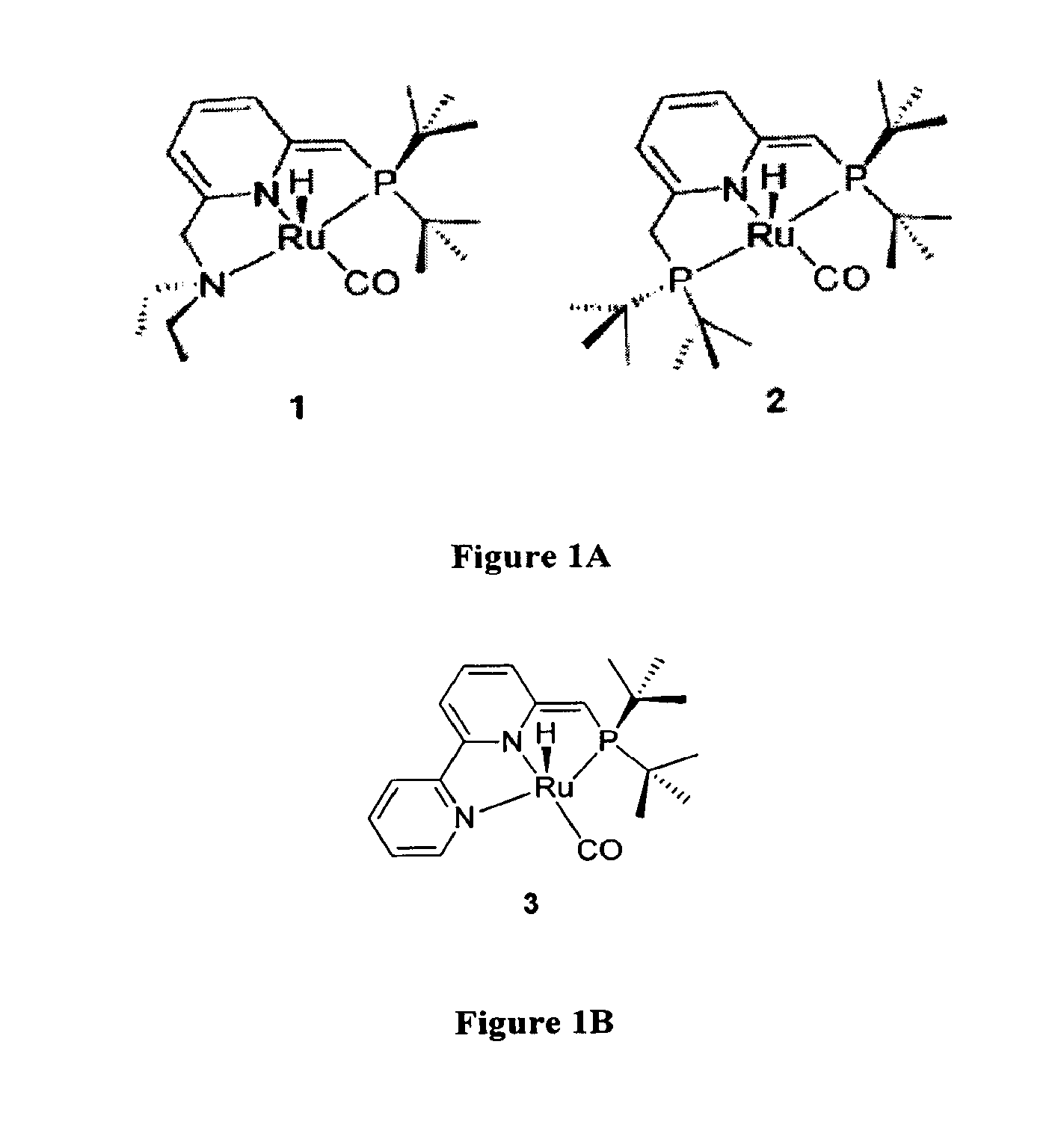
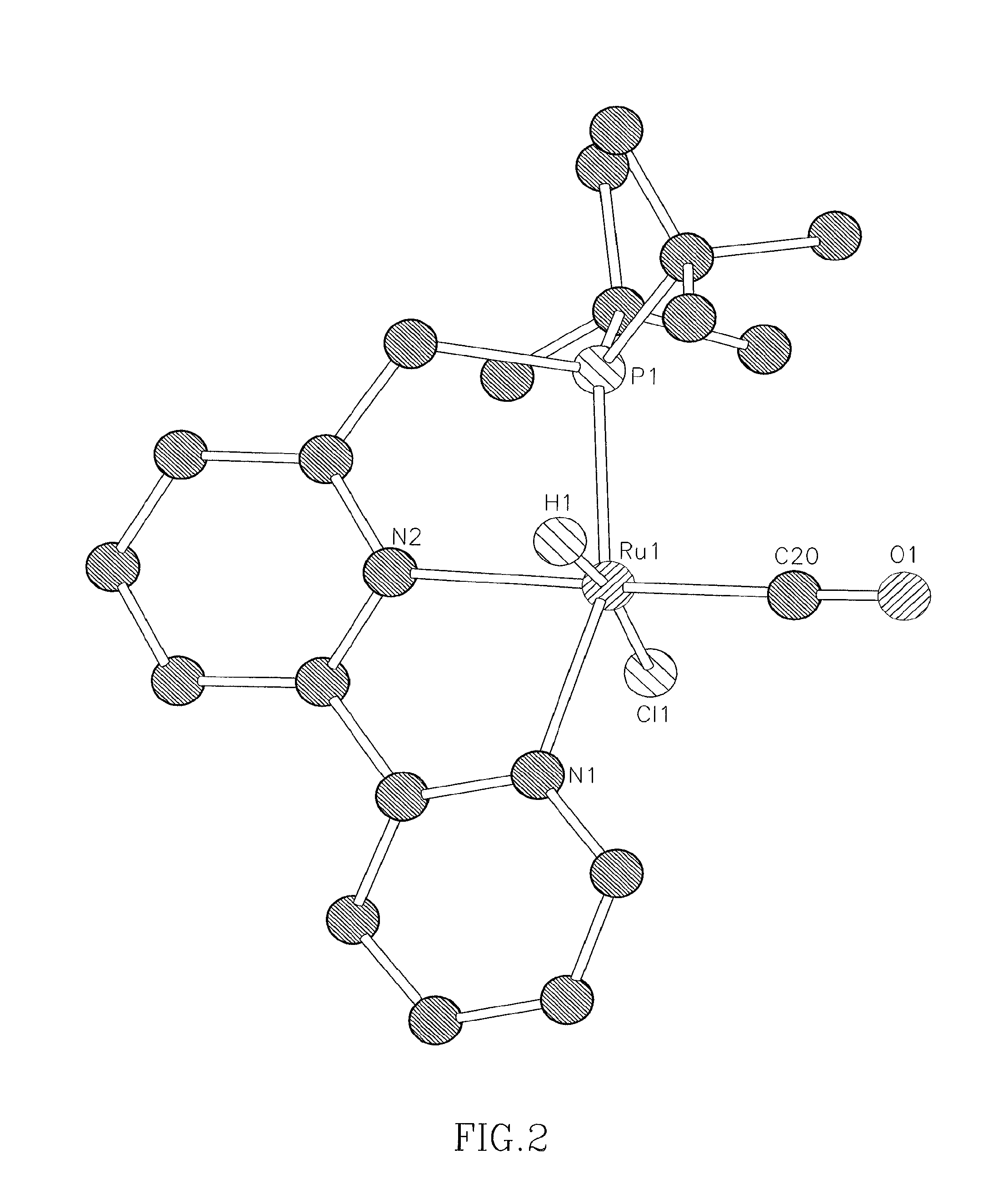
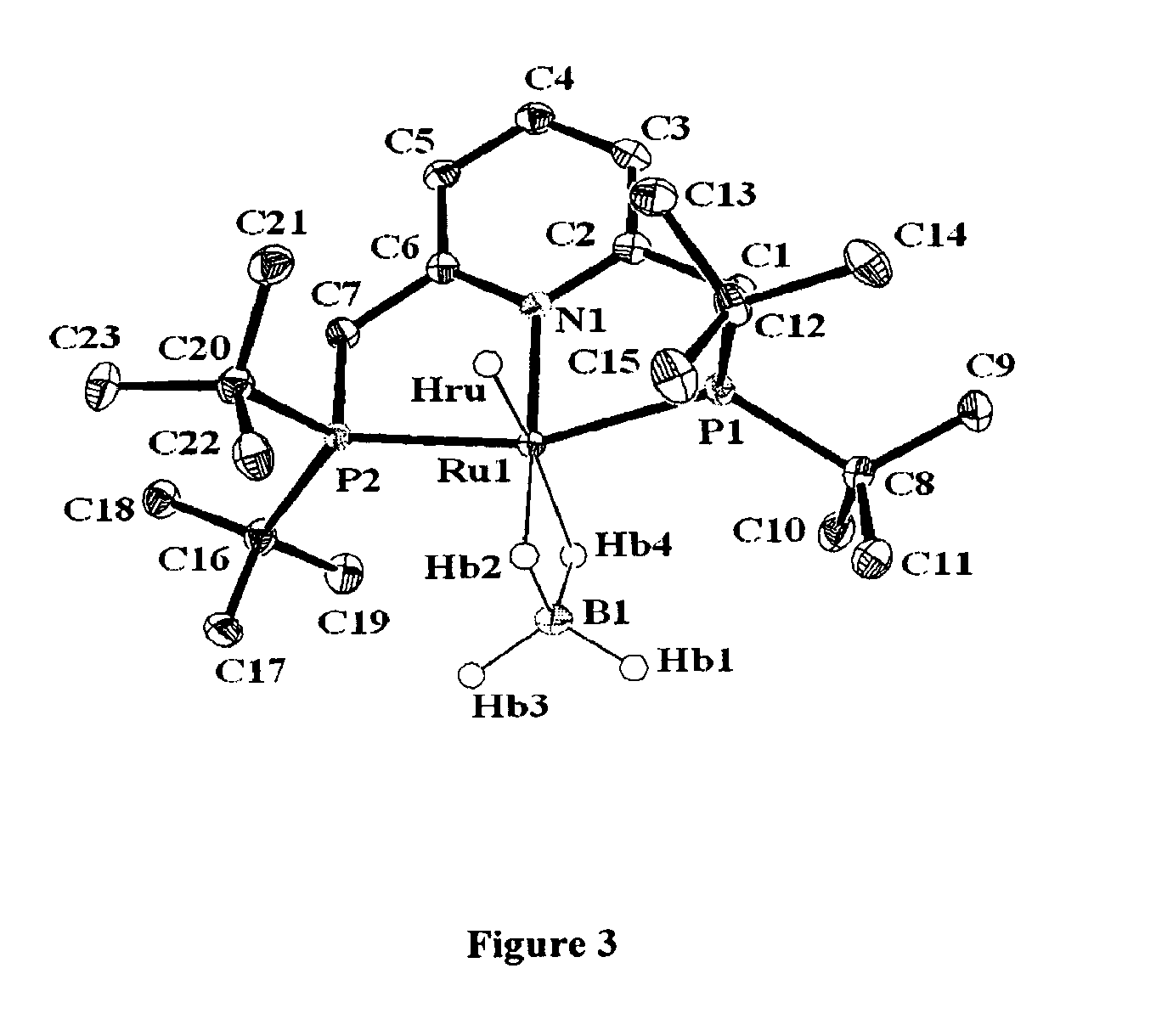
Novel ruthenium complexes and their uses in processes for formation and/or hydrogenation of esters, amides and derivatives thereof
a technology of ruthenium complexes and borohydride complexes, which is applied in the field of new ruthenium complexes and related borohydride complexes, can solve the problems of low yield, low turnover rate, and difficulty in design of such a reaction, and achieve high yield and high turnover rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hydrogenation of Amides to the Corresponding Alcohols and Amines
[0240]Examples of processes involving the hydrogenation of amides to alcohol and amines are shown in Scheme 13:
[0241](a) Using catalyst 3: A 100 mL Fischer-Porter tube was charged under nitrogen with the catalyst 3 (0.01 mmol), an amide (1.0 mmol), and THF (2 mL). The nitrogen present in the Fischer-Porter (100 mL) was replaced by H2 (twice with 30 psi) at room temperature, then it was filled with H2 (10 atm). The solution was heated at 110° C. (bath temperature) with stirring for 48 hrs. After cooling to room temperature, the H2 was vented carefully and the products were determined by GC with m-xylene as internal standard, using a Carboxen 1000 column on a HP 690 series GC system.
[0242](b) Using complex 4+ Base (generation of catalyst 3 in situ): In an open air, a solution of amide (1.0 mmol) in THF (1.0 mL) was transferred via syringe into a Fischer-Porter tube (100 mL) contains 0.01 mmol of catalyst 4. Then, KOtBu (0...
example 2
Hydrogenation of Formate Esters to methanol and alcohols; Dimethyl Carbonate to Methanol; Esters and Lactones to Alcohols, and Hydrogenation of Polycarbonates
A. Hydrogenation of Formates / Carbonate:
[0249]A 100 mL Fischer-Porter tube was charged under nitrogen with catalyst 3 (0.01 mmol), formate ester (15.0 mmol) or dimethyl carbonate (10.0 mmol) and THF (2 mL). After the nitrogen present in the Fischer-Porter tube was replaced by H2 (twice with 30 psi) at room temperature, the tube was filled with H2 (10 atm). The solution was heated at 110° C. (bath temperature) with stirring for 36 hrs. After cooling to room temperature, excess H2 was vented carefully and the products were analyzed by GC with m-xylene as internal standard, using a Carboxen 1000 column on a HP 690 series GC system.
B. Hydrogenation of Methyl Formate and Dimethyl Carbonate under 50 atmospheres and very low catalyst loading (entries 6 and 10, respectively, Table 4): A 30 mL Teflon coated Iron-Fischer-Porter (Autoclave...
example 3
Dehydrogenative Coupling of Primary Alcohols to Esters
[0277]Some processes involving the coupling of alcohols to esters are shown in Scheme 21:
A. Typical Procedures for the Catalytic Dehydrogenative Coupling of Primary Alcohols (Table 12)
[0278](a) Complex 3 (0.01 mmol), an alcohol (10 mmol), and toluene (2 mL) were taken in a Schlenk flask under an atmosphere of purified nitrogen in a glove box. The flask was equipped with a condenser and the solution was refluxed with stirring in an open system under argon for 24 hrs. After cooling to room temperature, the consumption of starting material and the formation of ester were determined by GC with m-xylene as an internal standard using a Carboxen 1000 column on a HP 690 series GC system.
[0279](b) Complex 4 (0.01 mmol), an alcohol (10 mmol), KOtBu (0.01 mmol) and toluene (2 mL) were taken in a Schlenk flask under an atmosphere of purified nitrogen in a glove box. The flask was equipped with a condenser and the solution was refluxed with s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com