Compositions comprising functionalized carbon-based nanostructures and related methods
a carbon-based nanostructure and functional technology, applied in the direction of organic compounds/hydrides/coordination complexes, organic compounds, iron organic compounds, etc., can solve the problems of intrinsic limitation of thermal and/or electrochemical boundaries of devices, the labile nature of such chemical functionalities is significant drawback to the current available technology, and the material generally cannot survive further thermal, electrochemical and/or chemical treatment of graphene-based materials. , to achieve the effect of reducing the amount of an
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
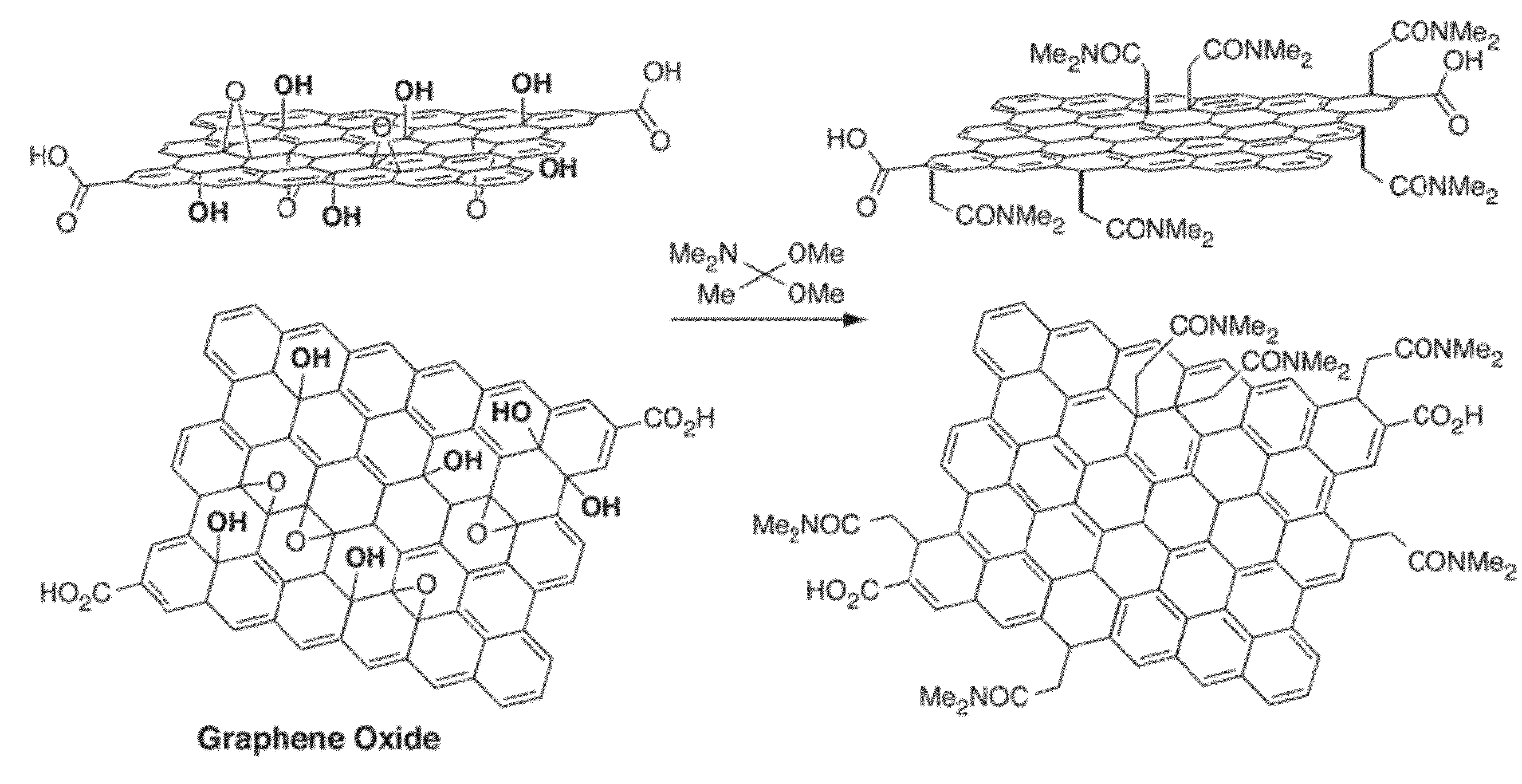
[0140]The following example describes the direct conversion of carbon-oxygen bonds on a graphite oxide basal plane to carbon bound carbonyl groups (e.g., see FIG. 10). GO (graphene oxide) in this example was functionalized using a Claisen rearrangement to allylically transpose tertiary alcohol functional groups found throughout the basal plane of graphite oxide into carbon-bound carbonyl derivatives. In traditional Claisen rearrangements, a vinyl allyl alcohol is heated to simultaneously break a carbon-oxygen bond while forming a carbon-carbon bond (via a sigmatropic rearrangement). In this example the tertiary alcohols that adorn the surface of graphite oxide function as the tertiary allylic alcohols. Direct treatment of these functional groups with a vinyl group equivalent (e.g., dimethylacetamide dimethylacetal is shown in FIG. 10) forms a vinyl allyl alcohol on GO, in situ. Heating this modified GO then directly breaks the tertiary alcohol bond and allylically forms a new carbon...
example 2
[0145]The following example described characterization of functionalized nanostructures formed using and / or comprising certain compositions of the present invention.
[0146]Reaction conditions for this example are substantially similar as described in Example 1, except for variations in the solvent and temperature. In addition, control reactions were carried out (e.g., wherein no DMADMA was added to the reaction mixture).
[0147]In this example, the following abbreviations are employed, and are further described herein:
rGO1—reaction in THF at 60° C., DMADMA added
rGO1c—control reaction in THF at 60° C., no DMADMA added
rGO2—reaction in 1,4-dioxane at 100° C., DMADMA added
rGO2c—control reaction in 1,4-dioxane at 100° C., no DMADMA added
rGO3—reaction in diglyme at 150° C., DMADMA added
rGO3c—control reaction in diglyme at 150° C., no DMADMA added)
rGO3b—reaction in diglyme sonicated for 3 h at pH=9
rGO3b (500)—sample annealed in 500° C. for 6 h in N2 atmosphere
[0148]Characterization: Throughou...
example 3
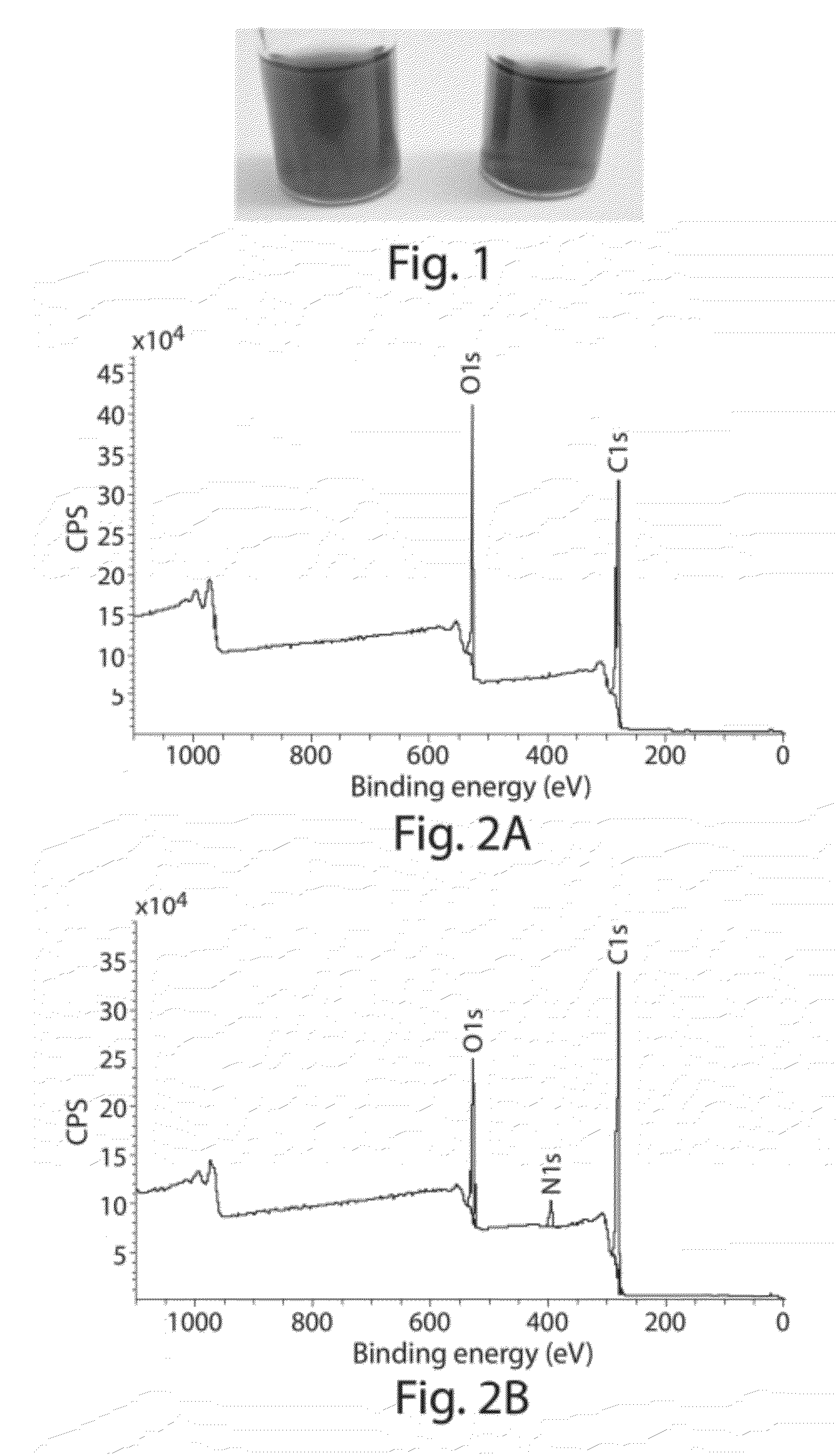
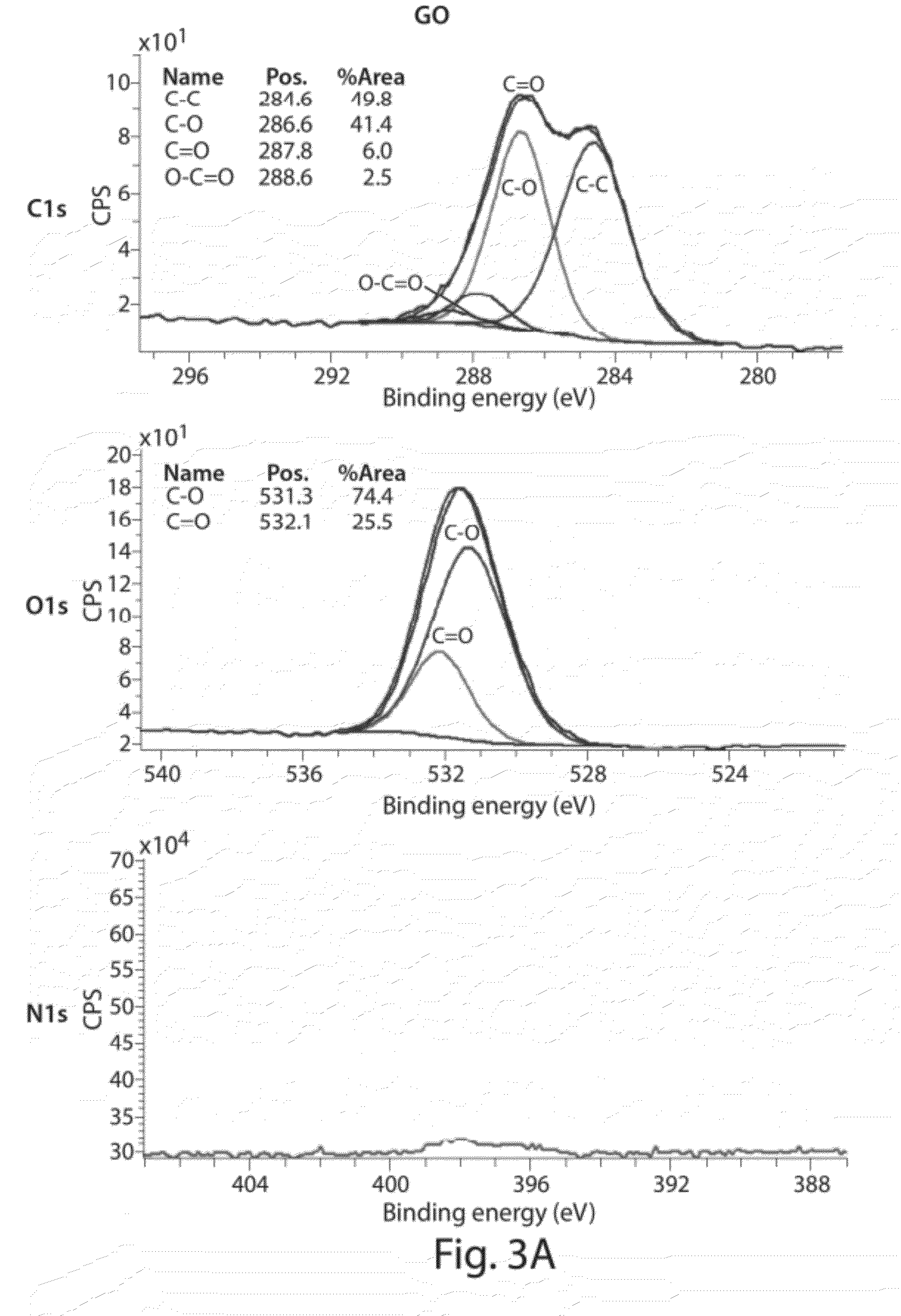
[0156]The following example describes the synthesis of allylic amide-functionalized graphene. (FIGS. 11A-B). As shown in FIG. 11C, after 1 hour of reacting graphene oxide with [(CH3)2N]C(OCH3)2CH3, the solution turns black in color. Upon completion of the reaction, UV-vis data of the resulting material indicated re-establishment of a conjugated network, and an FTIR spectrum indicated the presence of amide groups by the appearance of amide C═O stretching bands. (FIG. 11D) XRD data for graphene, graphene oxide, and allylic amide-functionalized graphene oxide is shown in FIG. 11E. XPS plots for graphene oxide, and allylic amide-functionalized graphene oxide confirmed the presence of amide functionality on the graphene surface (dialkylamides on activated carbon=399 / 9 eV). (FIGS. 11F-G) Furthermore, TGA data indicated that the functionalization was covalent in nature and that by increasing the reaction temperature, C—O functionalization decreased and C—C functionalization increased.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com