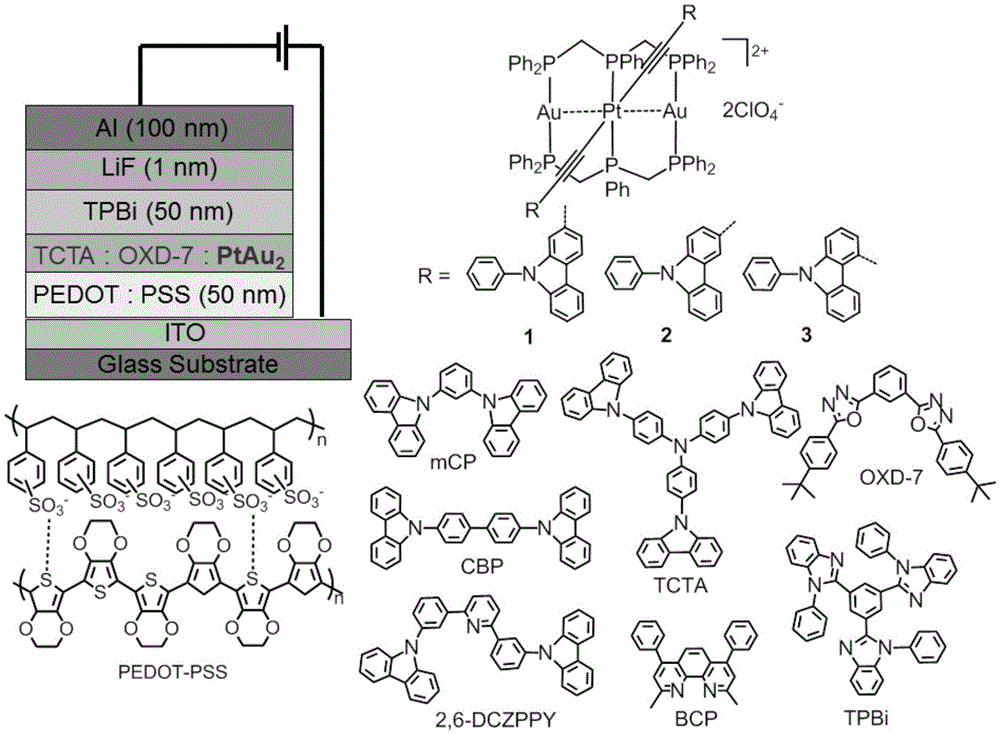
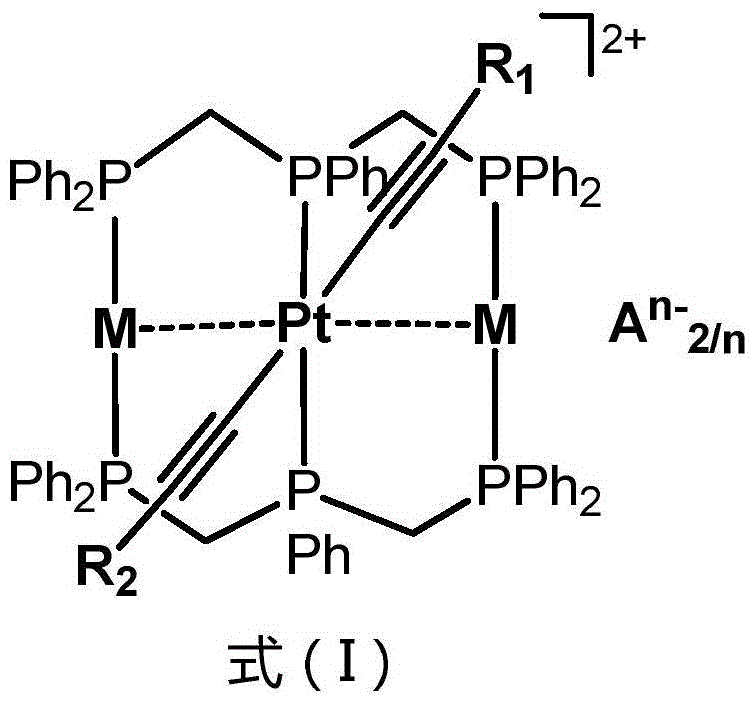
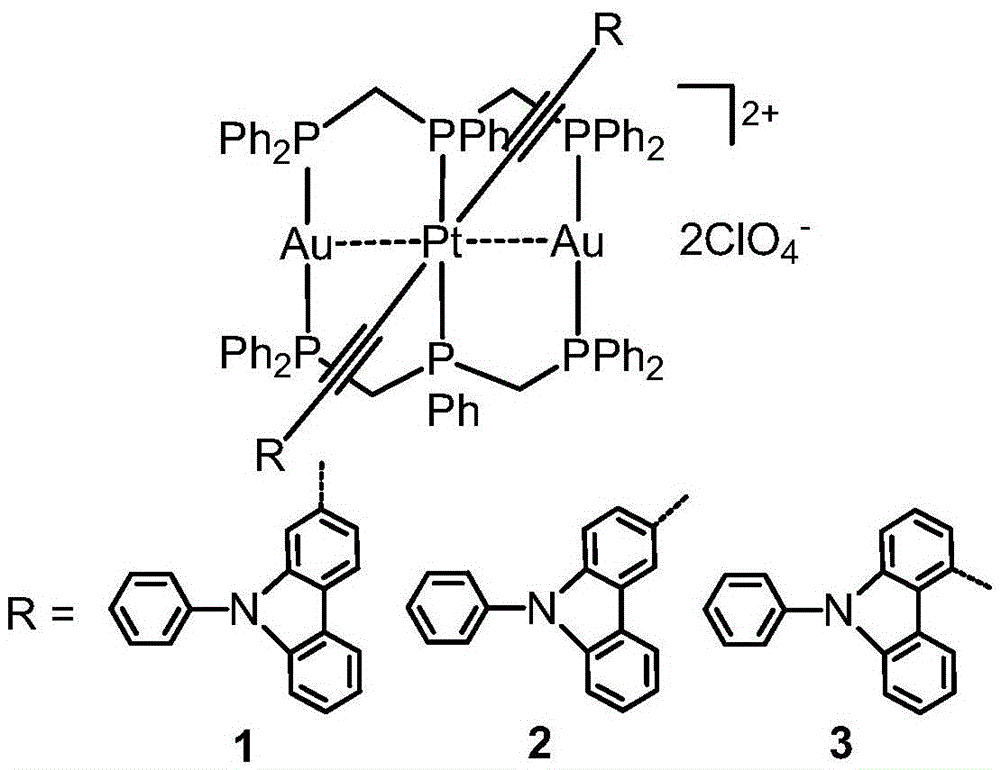
High-performance organic light-emitting diode
A light-emitting diode, organic technology, applied in the direction of light-emitting materials, organic chemistry, platinum group organic compounds, etc., can solve the problem of not breaking 16%, restricting the commercial application of related devices, etc., to reduce the preparation cost, improve the energy transfer efficiency, high The effect of electro-optical conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: Complex [PtAu 2 (dpmp) 2 (C≡C-2-(9-Ph)carb) 2 ](ClO 4 ) 2 (1) Preparation
[0047] [Au(tht) was dissolved in 20mL 2 ]ClO 4 (47.3mg, 0.1mmol) in dichloromethane solution was added Ph 2 P(CH 2 PPh 2 ) 2 (50.6 mg, 0.1 mmol). After stirring for 30 minutes, Pt(PPh 3 ) 2 (C≡C-2-(9-Ph)carb) 2 (62.5mg, 0.05mmol) in dichloromethane (5mL). The reaction solution turned yellow-green after being stirred at room temperature for 4 hours. The product was purified by silica gel column chromatography using CH 2 Cl 2 -MeCN(8:1) was the eluent to collect the yellow-green product. Yield: 83%. Elemental analysis (C 104 h 82 Au 2 Cl 2 N 2 o 8 P 6 Pt) Calculated: C, 53.53; H, 3.54; N, 1.20. Measured: C, 53.86; H, 3.62; 4 ] + ,1066.5(28)[M-2ClO 4 ] 2+ .Proton NMR spectrum (CDCl 3 ,ppm):8.18-8.14(d,2H,J=7.9Hz),7.96-7.92(d,2H,J=7.9Hz),7.87-7.79(m,6H),7.78-7.66(m,10H), 7.65-7.48(m,18H),7.47-7.41(t,6H,J=7.4Hz),7.39-7.29(m,6H),7.28-7.21(m,4H),7.20-7.06(m,...
Embodiment 2
[0048] Embodiment 2: Complex [PtAu 2 (dpmp) 2 (C≡C-3-(9-Ph)carb) 2 ](ClO 4 ) 2 (2) Preparation.
[0049] The preparation method is basically the same as the method in Example 1, only using Pt(PPh 3 ) 2 (C≡C-3-(9-Ph)carb) 2 Instead of Pt(PPh 3 ) 2 (C≡C-2-(9-Ph)carb) 2 . Yield: 85%. Elemental analysis (C 104 h 82 Au 2 Cl 2 N 2 o 8 P 6 Pt 1 / 2CH 2 Cl 2 ) Calculated: C, 52.82; H, 3.52; N, 1.18. Measured: 52.76; H, 3.37; N, 1.24. Electrospray mass spectrum (%): 2233.4 (100) [M-ClO 4 ] + ,1067.2(46)[M-2ClO 4 ] 2+ . H NMR spectrum (CDCl 3 ,ppm):8.04-7.90(m,10H),7.75-7.65(m,12H),7.64-7.57(m,18H),7.55-7.51(m,10H),7.47-7.40(m,12H),7.30 -7.24(t, 4H, J=7.9Hz), 7.14-7.07(t, 6H, J=7.3Hz), 6.86-6.80(m, 2H), 5.48-5.34(m, 4H), 4.63-4.47(m ,4H). Nuclear Magnetic Resonance Phosphorus Spectrum (CDCl 3 ,ppm):31.2(m,2P,J P-P =30.3Hz), 6.0(m,1P,J P-P =30.2Hz,J Pt-P = 2562Hz). Infrared spectrum (KBr, cm -1 ): 2098(w), 1100(s).
Embodiment 3
[0050] Embodiment 3: Complex [PtAu 2 (dpmp) 2 (C≡C-4-(9-Ph)carb) 2 ](ClO 4 ) 2 (3) Preparation.
[0051] The preparation method is basically the same as the method in Example 1, only using Pt(PPh 3 ) 2 (C≡C-4-(9-Ph)carb) 2 Instead of Pt(PPh 3 ) 2 (C≡C-2-(9-Ph)carb) 2 . The obtained product was dissolved in dichloromethane, n-hexane was slowly added dropwise to the solution, and the product was crystallized at room temperature and kept in the dark for 5 days. Yield: 80%. Elemental analysis (C 104 h 82 Au 2 Cl 2 N 2 o 8 P 6 P·C 6 h 14 ) calcd: C, 54.60; H, 4.00; N, 1.16. Measured values: C, 54.83; H, 3.92; N, 1.08. Electrospray mass spectrum m / z (%): 2233.3 (100) [M-ClO 4 ] + ,1067.1(37)[M-2ClO 4 ] 2+ . H NMR spectrum (CDCl 3 ,ppm):8.11-8.05(d,2H,J=8.0Hz),7.86-7.80(m,8H),7.70-7.65(m,10H),7.44-7.38(m,18H),7.37-7.30(m ,20H),6.96-6.86(m,10H),6.60-6.53(t,4H,J=7.5Hz),6.52-6.48(s,2H),5.23-4.98(m,4H),4.14-4.00(m ,4H). Phosphorus NMR spectrum (CDCl 3 ,ppm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com