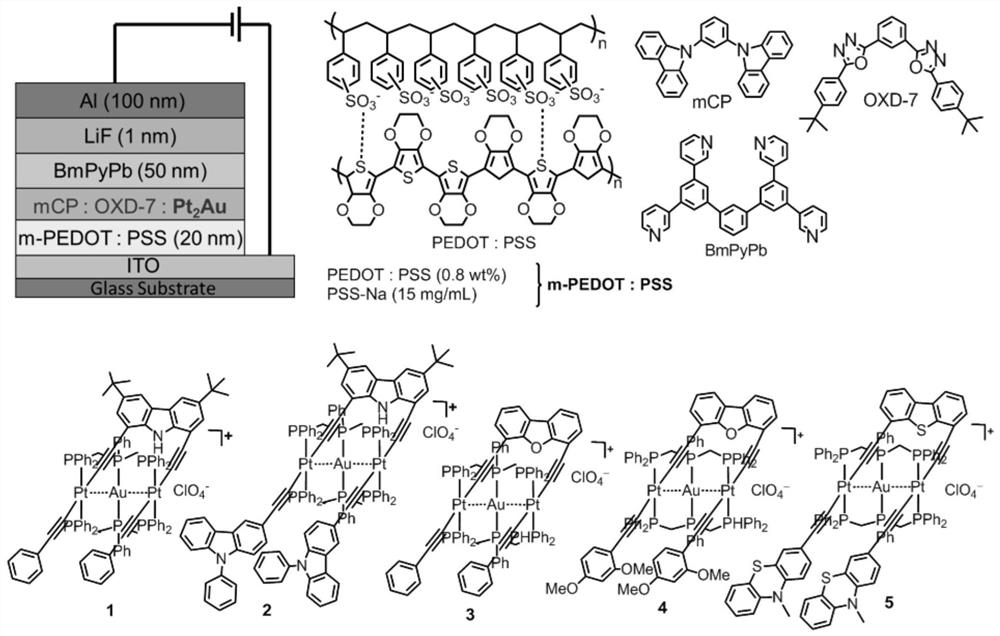
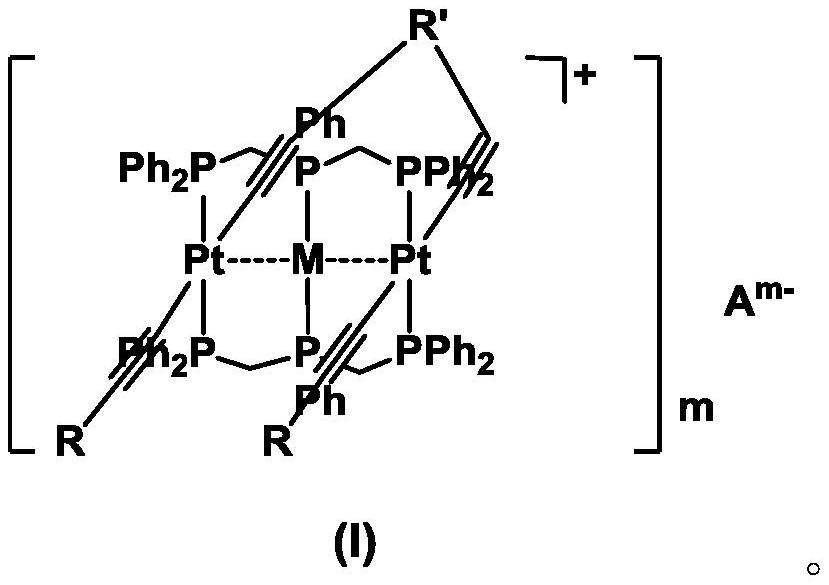
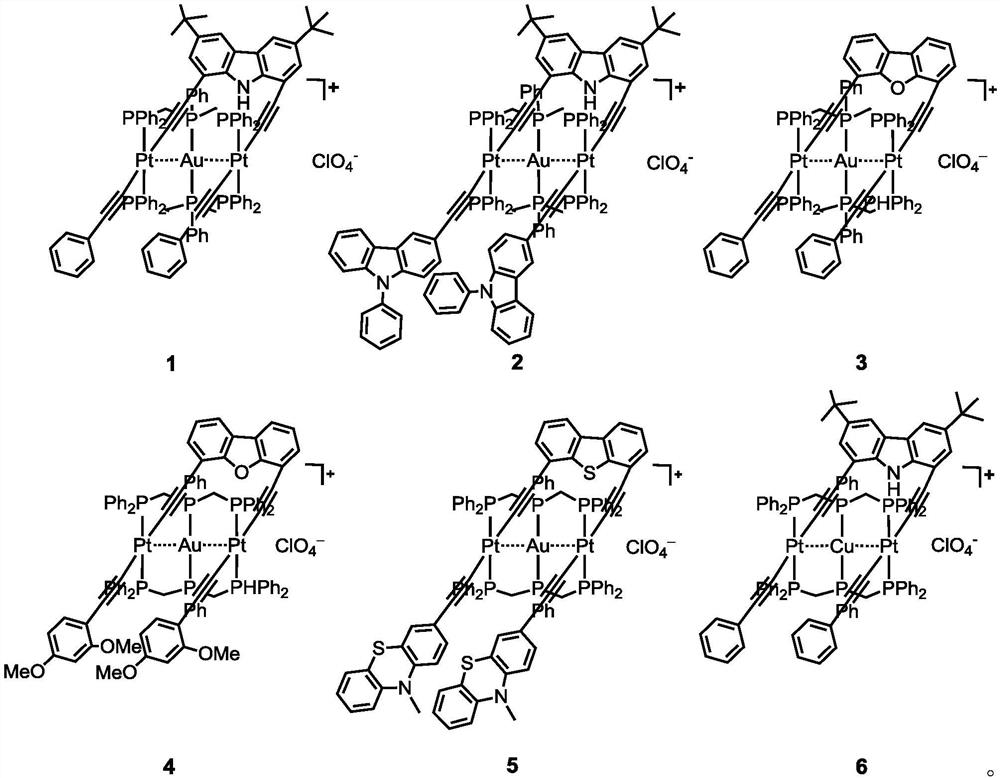
A kind of heterotrinuclear metal organic alkyne complex and its preparation method and application
A metal-organic and complex technology, applied in platinum-based organic compounds, platinum-group organic compounds, organic chemistry, etc., can solve the problems of shortage of iridium resources, incomplete chromaticity, high price, etc., and achieve the improvement of electroluminescence efficiency of devices. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Pt 2 Complex Pt 2 (PPh 3 ) 4 (μ-decz)(C≡CPh) 2 preparation of
[0066] Add Pt(PPh 3 ) 2 (C≡CPh)Cl (180mg, 0.21mmol), cuprous iodide (1mg), triethylamine (1mL) were reacted at 50°C for 18 hours. A clear yellow solution was obtained. After the reaction solution was concentrated, the product was purified by silica gel column chromatography, using petroleum ether-dichloromethane (2:1) as the eluent to collect a light yellow product with a yield of 78%. Elemental Analysis C 112 h 93 NP 4 Pt 2 Calculated: C, 68.39; H, 4.77. Measured: C, 68.14; H, 4.82. High resolution mass m / z (%): 1967.5549 (100) [M+H] +(calculated value 1967.5627). H NMR spectrum (400MHz, CD 2 Cl 2 ,ppm):7.85-7.77(m,24H),7.60(m,2H),7.32(t,24H,J=7.36),7.24(t,12H,J=7.32),6.94-6.88(m 6H), 6.73-6.70(m,2H),6.69(s,1H),6.30-6.22(m,4H),1.29(s,18H). Nuclear Magnetic Resonance Phosphorus Spectrum (162MHz,CD 2 Cl 2 ,ppm): 17.9(J Pt-P =2641Hz). Infrared spectrum (KBr, cm -1 ):2109m(C≡C). ...
Embodiment 2
[0067] Example 2: Pt 2 Complex Pt 2 (PPh 3 ) 4 (μ-decz)(C≡C-(9-Ph-carb-3)) 2 preparation of
[0068] The preparation method is basically the same as the method in Example 1, using Pt(PPh 3 ) 2 (C≡C-(9-Ph-carb-3)Cl instead of Pt(PPh 3 ) 2 (C≡CPh)Cl, productive rate: 72%. Elemental analysis C 136 h 107 N 3 P 4 Pt 2 Calculated: C, 71.10; H, 4.69. Measured: C, 70.99; H, 4.81. High-resolution mass spectrum m / z (%): 2297.6781 (100) [M+H] + (calculated value 2297.6793). H NMR spectrum (CD 2 Cl 2 ,ppm):7.95-7.80(m,24H),7.64-7.53(m,6H),7.51-7.39(m,12H),7.39-7.32(m,24H),7.31-7.21(m,12H),6.97 (d, 2H, J=8.48), 6.85(s, 1H), 6.74(s, 2H), 6.33(d, 2H, J=8.44), 1.29(s, 18H). Nuclear Magnetic Resonance Phosphorus Spectrum (162MHz, CD 2 Cl 2 ,ppm): 18.0(J Pt-P =2645Hz). Infrared spectrum (KBr, cm -1 ):2117(w).
Embodiment 3
[0069] Example 3: Pt 2 Complex Pt 2 (PPh 3 ) 4 (μ-debf)(C≡CPh) 2 preparation of
[0070] The preparation method is basically the same as the method in Example 1, using debf-2H to replace decz-2H, productive rate: 70%. Elemental analysis C 104 h 76 OP 4 Pt 2 Calculated: C, 67.31; H, 4.13. Measured: C, 67.21; H, 4.08. High-resolution mass spectrum m / z (%): 1854.4132 (100) [M+H] + (calculated value 1854.4140). H NMR spectrum (CD 2 Cl 2 ,ppm):7.72-7.68(m,26H),7.61-7.57(m,4H),7.36-7.32(m,26H),7.27-7.24(m,14H),6.97-6.92(t,2H,J= 8.38), 6.53-6.51(d, 2H, J=8), 6.46-6.45(d, 2H, J=8.38). Nuclear Magnetic Resonance Phosphorus Spectrum (162MHz, CD 2 Cl 2 ,ppm): 17.77(J Pt-P =2134Hz). Infrared spectrum (KBr, cm -1 ):2110(w).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com