Phosphorescence material iridium metal complex, preparation method and organic electroluminescent device
An iridium metal complex, organic technology, applied in the direction of luminescent materials, electrical solid devices, organic chemistry, etc., can solve the problems of low efficiency and difficulty in obtaining satisfactory luminescent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
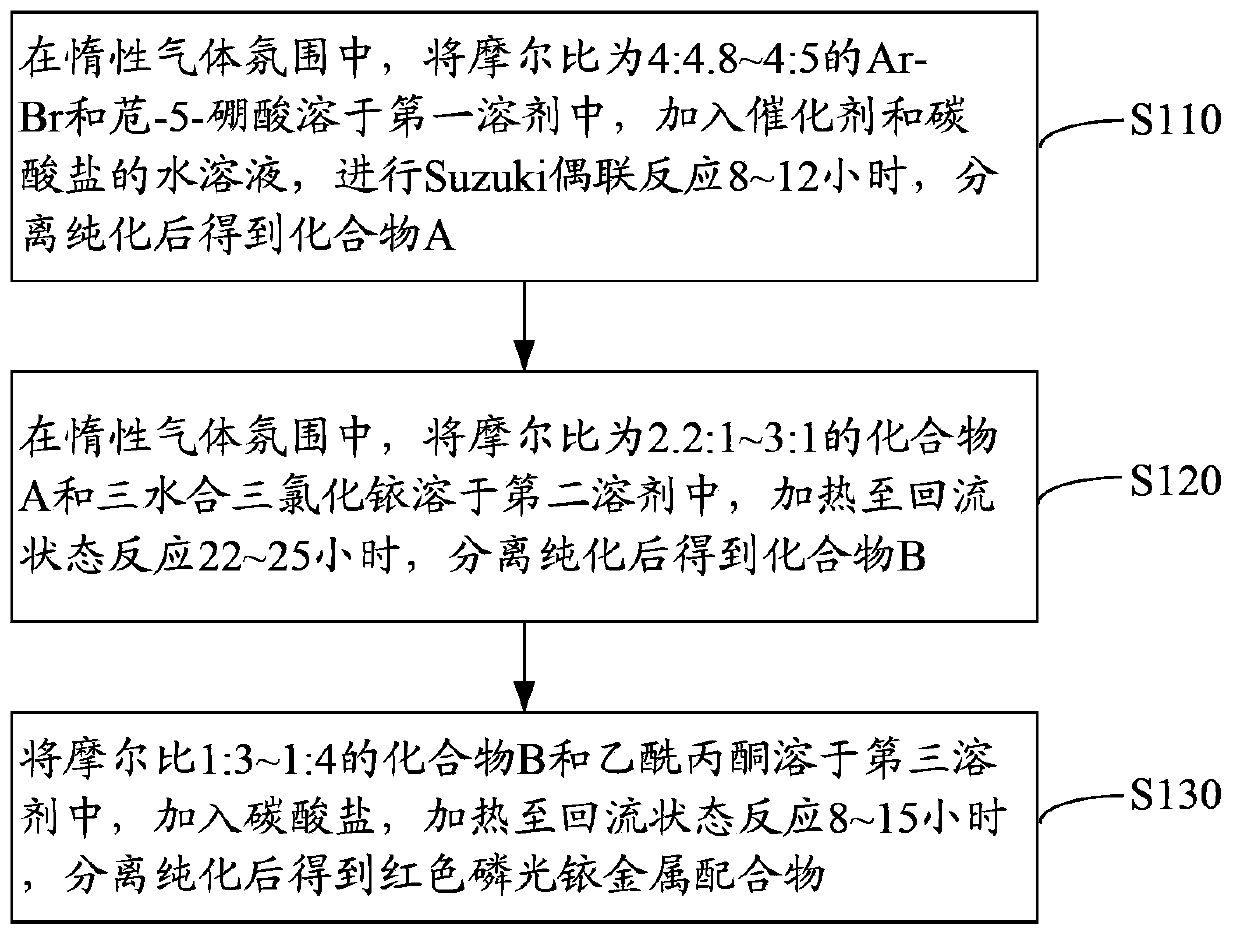
[0034] see figure 1 , the preparation method of the red phosphorescence iridium metal complex of an embodiment, comprises the steps:
[0035] Step S110: In an inert gas atmosphere, dissolve Ar-Br and acenaphthyl-5-boronic acid at a molar ratio of 4:4.8 to 4:5 in the first solvent, add a catalyst and an aqueous carbonate solution, and perform Suzuki coupling React for 8 to 12 hours, obtain compound A after separation and purification, and the structural formula of compound A is Among them, -Ar is or
[0036] The inert gas is argon, helium or neon.
[0037] The first solvent is toluene, tetrahydrofuran (THF) or N,N-dimethylformamide. The amount of the first solvent is suitable to fully dissolve Ar-Br and acenaphthylene-5-boronic acid. Preferably, the solid-to-liquid ratio of acenaphthyl-5-boronic acid to the first solvent is 4.8mmol:25mL˜4.8mmol:20mL.
[0038] The catalyst is an organic palladium catalyst, specifically tetrakistriphenylphosphine palladium (Pd (pph 3 )...
Embodiment 1
[0069] Red phosphorescent bis[2-(acenaphth-5-yl)quinoline-N,C 2 Synthesis of '](acetylacetonate) iridium complexes
[0070] Red phosphorescent bis[2-(acenaphth-5-yl)quinoline-N,C 2 '] (acetylacetonate) the structural formula of iridium complex is as follows:
[0071]
[0072] (1) Synthesis of 2-(acenaphthyl-5-yl)quinoline
[0073]
[0074] Under the protection of argon, 0.83g (4mmol) 2-bromoquinoline, 0.95g (4.8mmol) acenaphthene-5-boronic acid and 0.28g (0.24mmol) tetrakistriphenylphosphine palladium were dissolved in 25mL THF, and then poured into the reaction system 10 mL of an aqueous solution containing 1.10 g (8 mmol) of potassium carbonate was added dropwise to the solution, heated, and stirred under reflux for 10 h. After the reaction was complete, it was naturally cooled to room temperature, and extracted three times with 15 mL of water and 15 mL of ethyl acetate successively. The organic phase was dried with anhydrous magnesium sulfate, filtered, and the so...
Embodiment 2
[0102] Red Phosphorescent Bis[1-(Acenaphth-5-yl)isoquinoline-N,C 2 Synthesis of '](acetylacetonate) iridium complexes
[0103] Red Phosphorescent Bis[1-(Acenaphth-5-yl)isoquinoline-N,C 2 '] (acetylacetonate) the structural formula of iridium complex is as follows:
[0104]
[0105] (1) Synthesis of 1-(acenaphthyl-5-yl)isoquinoline
[0106]
[0107] Under the protection of argon, 0.83g (4mmol) 1-bromoisoquinoline, 0.99g (5mmol) acenaphthene-5-boronic acid and 0.23g (00.20mmol) tetrakistriphenylphosphine palladium were dissolved in 25mL THF, and then poured into the reaction system 10 mL of an aqueous solution containing 1.38 g (10 mmol) of potassium carbonate was added dropwise. Heated and stirred under reflux for 12h. After the reaction was complete, it was naturally cooled to room temperature, and extracted three times with 15 mL of water and 15 mL of ethyl acetate successively. The organic phase was dried over anhydrous magnesium sulfate and filtered. The solvent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com