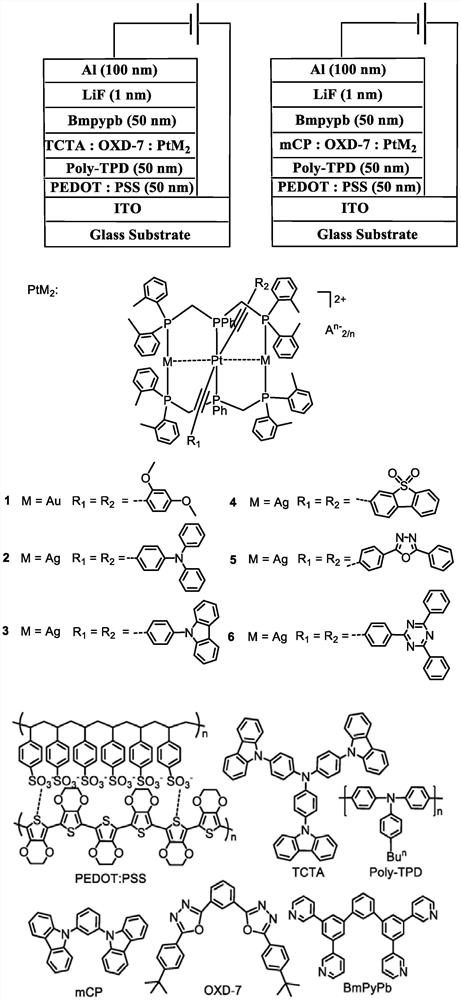
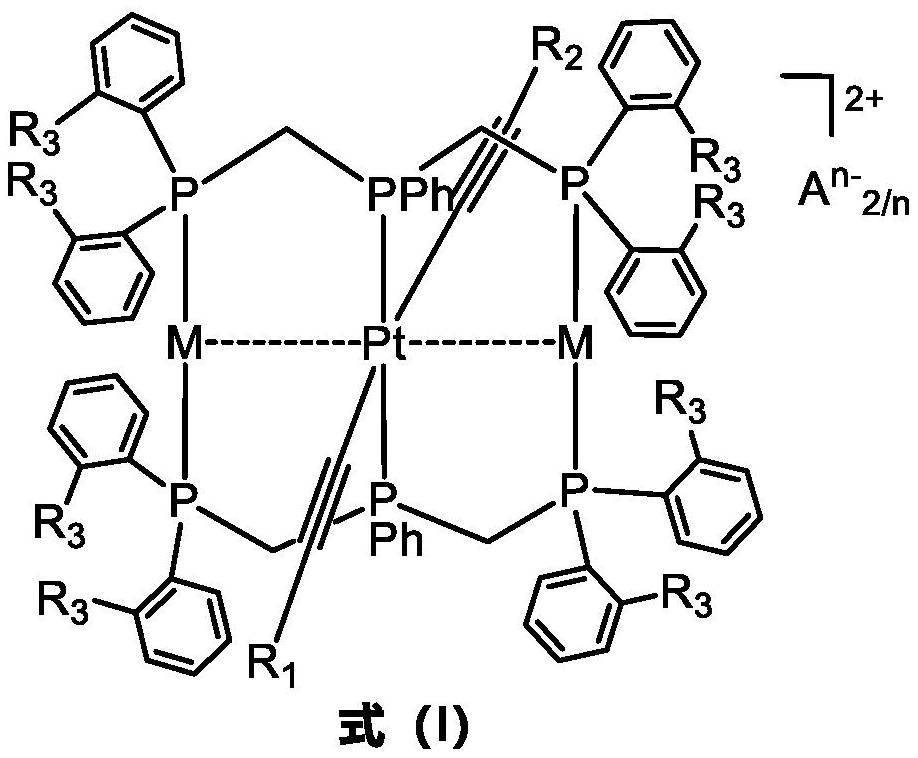
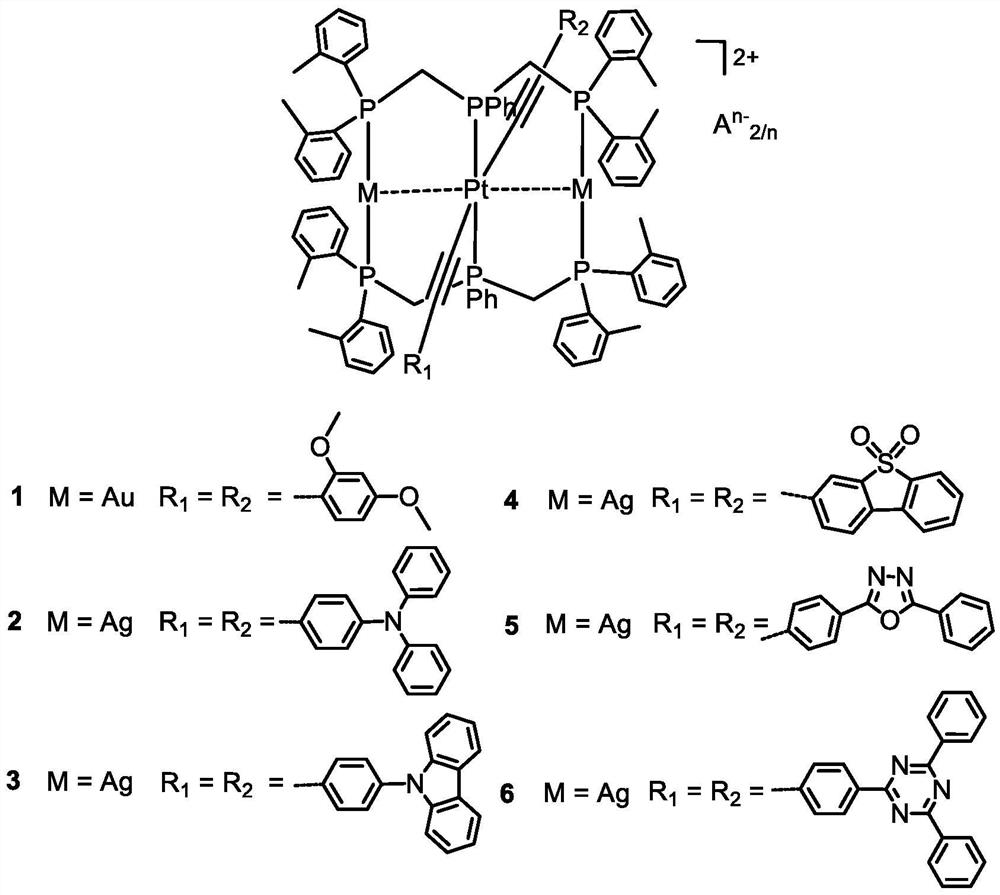
phosphorescent ptm 2 (m = Cu, Ag, Au) complexes and their organic light-emitting diodes
A technology of light-emitting diodes and complexes, applied in the field of novel PtM2 heterotrinuclear complex light-emitting materials and organic light-emitting diodes, can solve the problems of difficult solution printing and film formation, and achieve improved energy transfer efficiency, improved luminous color purity, maximum The effect of high current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: Complex [PtAu 2 (dTolmp) 2 (C≡CC 6 h 3 (OMe) 2 -2,4) 2 ](CF 3 SO 3 ) 2 (1) Preparation
[0063] [Au(tht) was dissolved in 20mL 2 ](CF 3 SO 3 ) (0.1 mmol) in dichloromethane was added dTolmp (0.1 mmol). After stirring for 30 minutes, Pt(PPh 3 ) 2 (C≡CC 6 h 3 (OMe) 2 -2,4) 2 (0.05 mmol) in dichloromethane (5 mL). The reaction was stirred at room temperature for 4 hours. The product was purified by silica gel column chromatography using CH 2 Cl 2 -MeCN (8:1) was collected as eluent to give pure product. Yield: 83%. High resolution mass spectrometry [M-2CF 3 SO 3 ] 2+ Calculated value: 1018.2208, measured value: 1018.2207. H NMR spectrum (CD 2 Cl 2 ,ppm):8.12-8.09(m,4H),7.77-7.72(m,4H),7.37-7.31(m,8H),7.18-7.14(m,14H),6.98-6.91(m,8H),6.76 -6.72(t,4H),6.67-6.65(d,2H),6.43-6.37(m,4H),5.04-5.01(m,8H),3.90,3.86(s,12H),2.22, 2.13(s, 24H). Nuclear Magnetic Resonance Phosphorus Spectrum (CD 2 Cl 2 ,ppm):20.28(m,4P,J P–P =33.10Hz), 8.24...
Embodiment 2
[0064] Embodiment 2: Complex [PtAg 2 (dTolmp) 2 (C≡C-4-TPA) 2 ](ClO 4 ) 2 (2) Preparation.
[0065] [Ag(tht)](ClO 4 ) (0.1 mmol) in dichloromethane was added dTolmp (0.1 mmol). After stirring for 30 minutes, Pt(PPh 3 ) 2 (C≡C-4-TPA) 2 (0.05 mmol) in dichloromethane (5 mL). The reaction was stirred at room temperature for 4 hours. The product was purified by silica gel column chromatography using CH 2 Cl 2 -MeCN (8:1) was collected as eluent to give pure product. Yield: 78%. High resolution mass spectrometry [M-2ClO 4 ] 2+ Calculated value: 1036.2117, measured value: 1036.2098. H NMR spectrum (CD 2 Cl 2 , ppm):8.12-7.90(m,7H),7.65-7.58(m,4H),7.46-6.94(m,44H),6.88-6.58(m,12H), 6.39-6.25(m,3H),6.66 -6.62(t,2H),6.59-6.56(m,3H),6.43-6.41(d,1H),6.22-6.16(m,3H),4.73-4.04(m,8H),2.16-2.01(m, 24H). Nuclear Magnetic Resonance Phosphorus Spectrum (CD 2 Cl 2 ,ppm): 12.94,11.50(m,2P,J Pt–P =2442Hz,J Pt–P =2407Hz,J P–P =33.3Hz), -12.25, -16.10, -19.32 (m, 4P, J Ag–P ...
Embodiment 3
[0066] Embodiment 3: Complex [PtAg 2 (dTolmp) 2 (C≡C-4-Ph-9-Carb) 2 ](CF 3 SO 3 ) 2 (3) Preparation.
[0067] [Ag(tht)](ClO 4 ) (0.1 mmol) in dichloromethane was added dTolmp (0.1 mmol). After stirring for 30 minutes, Pt(PPh 3 ) 2 (C≡C-4-Ph-9-Carb) 2 (0.05 mmol) in dichloromethane (5 mL). The reaction was stirred at room temperature for 4 hours. The product was purified by silica gel column chromatography using CH 2 Cl 2 -MeCN (8:1) was collected as eluent to give pure product. Yield: 80%. High resolution mass spectrometry [M-2CF 3 SO 3 ] 2+ Calculated value: 1034.1960, measured value: 1034.1960. H NMR spectrum (CD 2 Cl 2 ,ppm):8.26-8.17(m,11H),7.83-7.71(m,4H),7.58-7.32(m,24H), 7.29-7.05(m,18H),7.00-6.93(m,4H),6.87 -6.73(m,4H),6.56-6.54(d,1H), 4.98-4.27(m,8H),2.31-2.12(m,24H). NMR phosphorous spectrum (CD 2 Cl 2 ,ppm): 12.93and 12.03(m,2P,J Pt–P =2431Hz,J Pt–P =2386Hz,J P–P =33.2Hz), -12.39, -16.31, -19.52 (m, 4P, J Ag–P =522Hz,J P–P =43.5Hz). Infrar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com