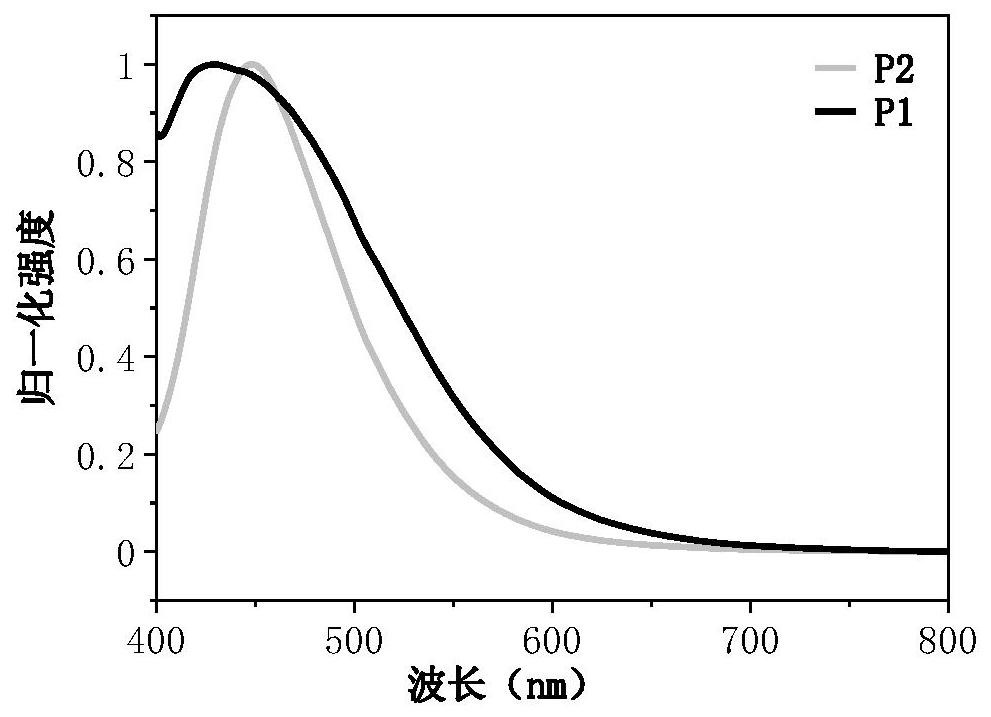
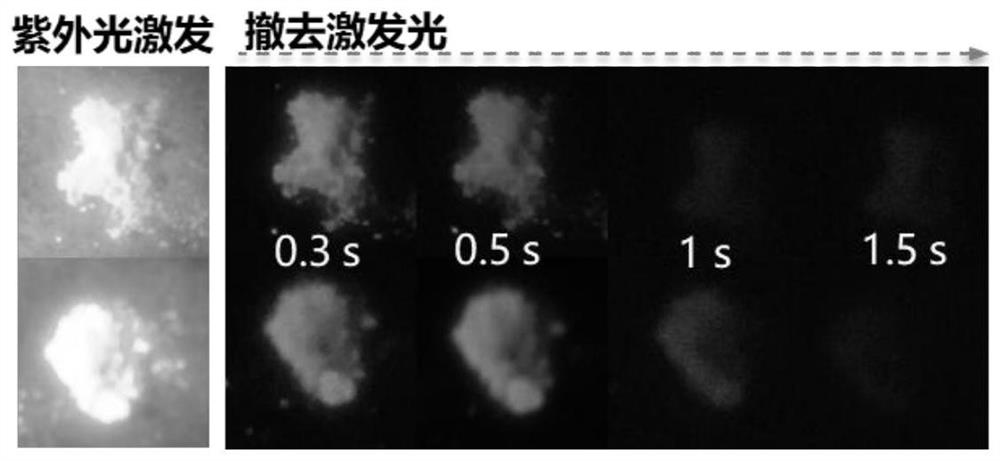
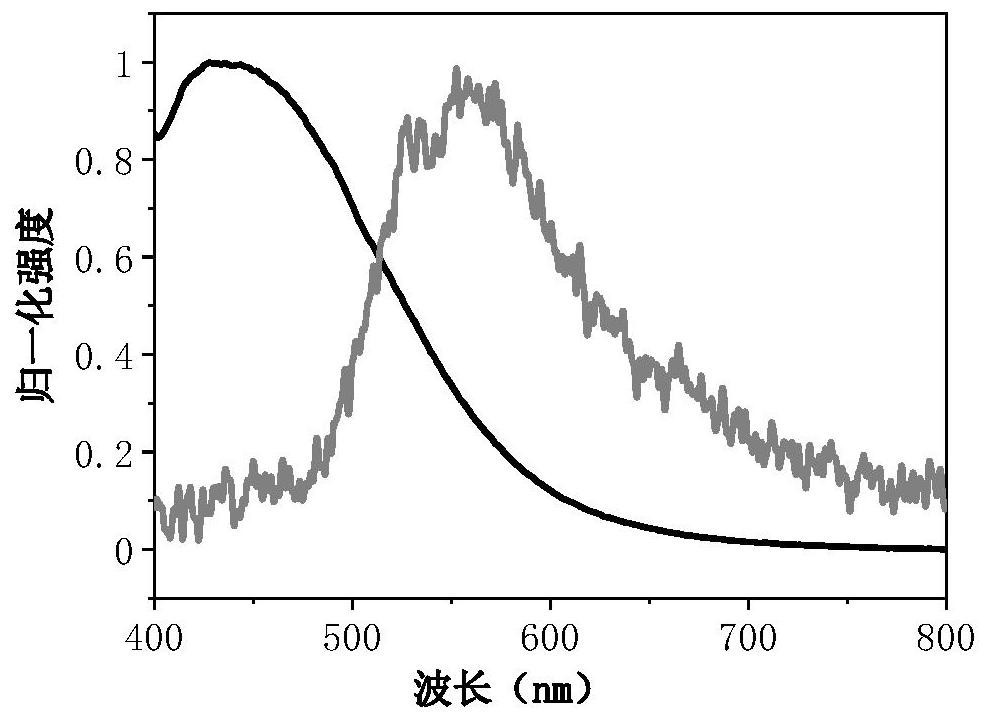
Organic long afterglow material containing triphenylphosphine (triphenylphosphine oxide) and preparation method and use method thereof
A long afterglow material, triphenyl technology, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of late start of research and development of organic long afterglow materials and limited types, and achieve long afterglow luminescence and easy purification. , the effect of suppressing non-radiative transitions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of intermediate [2-iodotriphenylamine]
[0032]
[0033]Under a nitrogen atmosphere, add diphenylamine (2.00g, 11.82mmol) into a 250mL dry three-necked flask, then add 1,2-diiodobenzene (4.68g, 14.18mmol), add 50mL toluene to dissolve, add o-phenanthroline ( 255.58mg, 1.42mmol), cuprous iodide (225.08mg, 1.18mmol) and potassium hydroxide (2.65g, 47.27mmol), heated to 110°C and stirred for 16h. After the reaction, the toluene was removed from the reaction solution by rotary evaporation, and 50 ml of dichloromethane was added to dissolve it, then the reaction solution was poured into a separatory funnel, and washed with water for 2-3 times. The organic layer was dried with anhydrous sodium sulfate, filtered, and the filtrate was spin-dried in a rotary evaporator, and then purified by silica gel column chromatography, and the eluent was a mixture of dichloromethane and n-hexane with a volume ratio of 1:2. solution. 2.00 g of yellow-white solid was obtain...
Embodiment 2
[0038] Synthesis of target product P2
[0039]
[0040] Add Example 1 (0.40 g, 0.93 mmol) into a round-bottomed flask, and dissolve in 20 ml of tetrahydrofuran. 6 mL of hydrogen peroxide aqueous solution (30%) was added, and after stirring for 5 hours, 50 mL of dichloromethane and 50 mL of water were added to the reaction liquid, followed by liquid separation. The dichloromethane layer was spin-dried with a rotary evaporator to obtain a white powder. The white powder was recrystallized from dichloromethane / n-hexane to obtain 0.35 g of white solid with a yield of 84.4%.
Embodiment 3
[0042] (1) Synthesis of intermediate 2-bromotriphenylphosphine
[0043]
[0044] With reference to step (2) in Example 1, 2-bromotriphenylphosphine was synthesized by using o-bromoiodobenzene instead of 2-iodotriphenylamine. (59.2% yield)
[0045] (2) Synthesis of intermediate 2-bromotriphenylphosphine oxide
[0046]
[0047] Referring to the steps in Example 2, 2-bromotriphenylphosphine oxide was synthesized by using 2-bromotriphenylphosphine instead of 2-dianilinotriphenylphosphine. (yield is 81.5%)
[0048] (3) Synthesis of target product 2-(2-diphenylamino)phenyltriphenylphosphine oxide
[0049]
[0050] Add 2-boronate triphenylamine (1.25g, 3.36mmol), 2-bromotriphenylphosphine oxide (1.00g, 2.80mmol) and sodium hydroxide (0.34g, 8.40mmol) into a 250mL three-necked flask, add Appropriate amount of ethylene glycol dimethyl ether, add a teaspoon of tetrakistriphenylphosphine palladium in a nitrogen environment. Raise the temperature to 110°C and react for 12h. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com