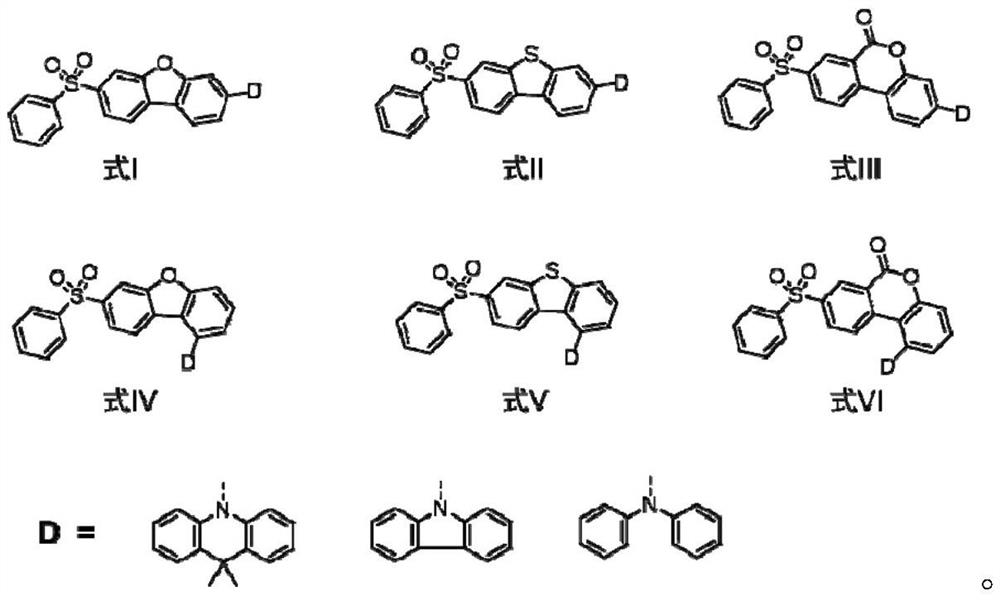
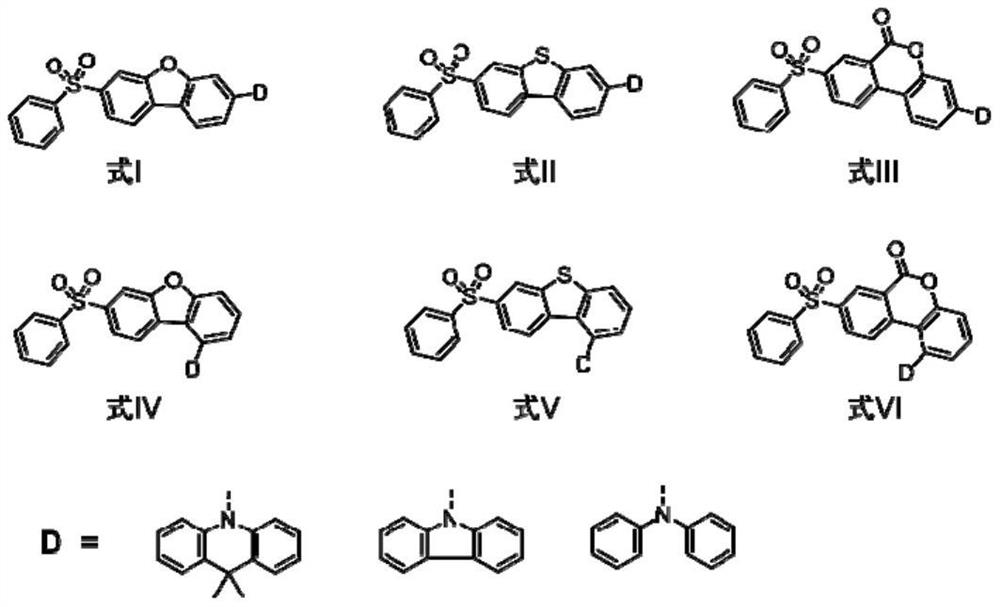
Blue thermal activity delayed fluorescent material based on dibenzoheterocycle conjugated pi bridge, and application thereof
A technology of delayed fluorescence and fluorescent materials, applied in the field of organic electroluminescent materials, which can solve the problems of increased non-radiative transitions, reduction of material luminous efficiency, and large increase in vibration intensity, to achieve the effect of suppressing non-radiative transitions and improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
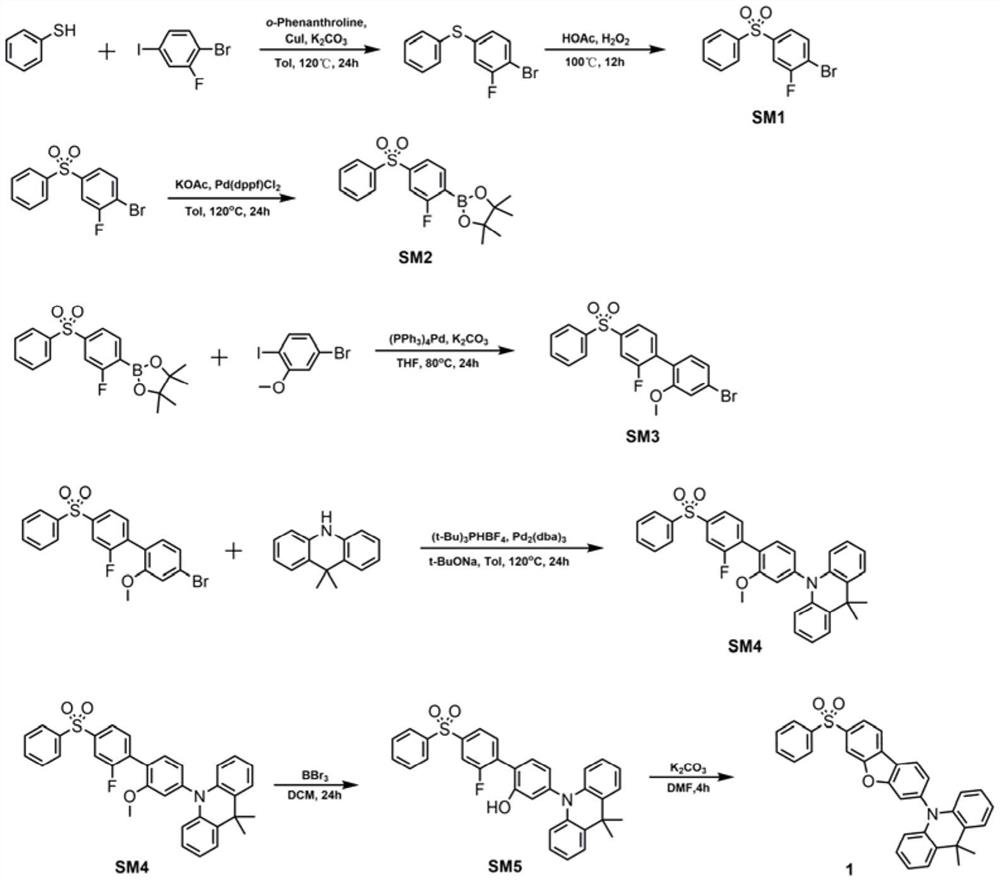
[0026] Taking formula I as an example, the synthesis scheme of TADF blue light material based on dibenzoheterocyclic conjugated π bridge is as follows:
[0027]
[0028] Synthesis of compound SM1
[0029] Add thiophenol (1.53g, 13.8mmol), 1-bromo-2-fluoro-4-iodobenzene (5g, 16.6mmol), cuprous iodide (0.53g, 2.8mmol), o- Phenanthroline (0.5 g, 2.8 mmol), sodium tert-butoxide (6.7 g, 69.2 mmol), and 100 mL of analytically pure toluene were heated to reflux at 120° C. for 24 hours under nitrogen protection. Cool to room temperature, remove toluene by rotary evaporation under reduced pressure, extract with dichloromethane (3×50mL), wash with water three times, dry over anhydrous magnesium sulfate, filter, collect the filtrate, spin dry the solvent, and use petroleum ether as the eluent for column chromatography 3.5 g of a colorless oily liquid were isolated with a yield of 89.5%. 1H NMR (300MHz, CDCl3) δ7.93(d, J=7.0Hz, 2H), 7.81(d, J=8.6Hz, 2H), 7.67–7.51(m, 4H).
[0030] A...
Embodiment 2
[0042] Compound 1 in Example 1 was crystallized from chloroform / methanol mixed solvent by solvent evaporation method, and its crystal structure was obtained by X-ray single crystal diffraction as follows: figure 1 shown. The single crystal structure of compound 1 shows that it is a dimer, and there are two conformations in the molecular structure, and the dihedral angles are 106.07° and 81.03°, respectively.
Embodiment 3
[0044] Compound SM4, SM5 and compound 1 in embodiment 1 are dissolved in toluene to form 10 -5 M solution, test the UV-Vis absorption and photoluminescence spectra of its solution. Depend on figure 1 It can be seen that there are roughly two absorption peaks in the UV-Vis absorption spectrum of the compound in solution: the absorption peak at the short wavelength (300nm) is mainly attributed to the π-π* transition absorption of the molecule; the absorption peak at the long wavelength (370nm) is attributed to Intramolecular charge transfer (ICT) transition absorption peak from donor unit to acceptor unit. like figure 2 As shown, the maximum emission peak of compound SM4 is 445nm, the emission peak of compound SM5 is 478nm, and the emission peak of compound 1 is 465nm, indicating that the three compounds are all in the blue light emission region.
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com