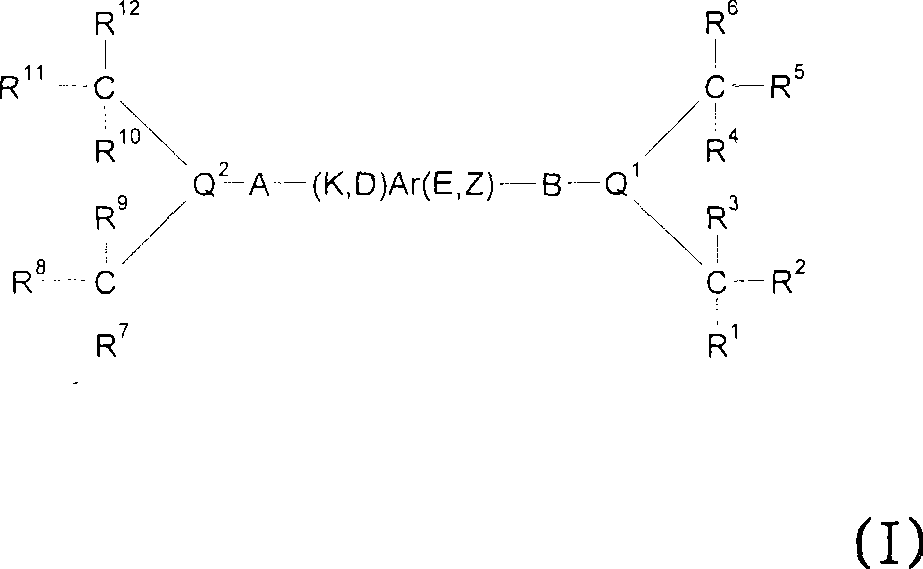
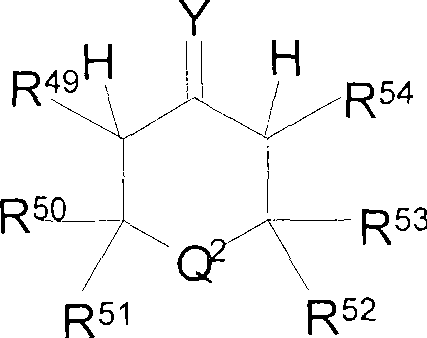
Metal complexes for use in the carbonylation of ethylenically unsaturated compounds
A technology of metal complexes and compounds, applied in platinum group organic compounds, metallocenes, compounds containing elements of Group 8/9/10/18 of the periodic table, etc., can solve the instability of ligand-metal complexes And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
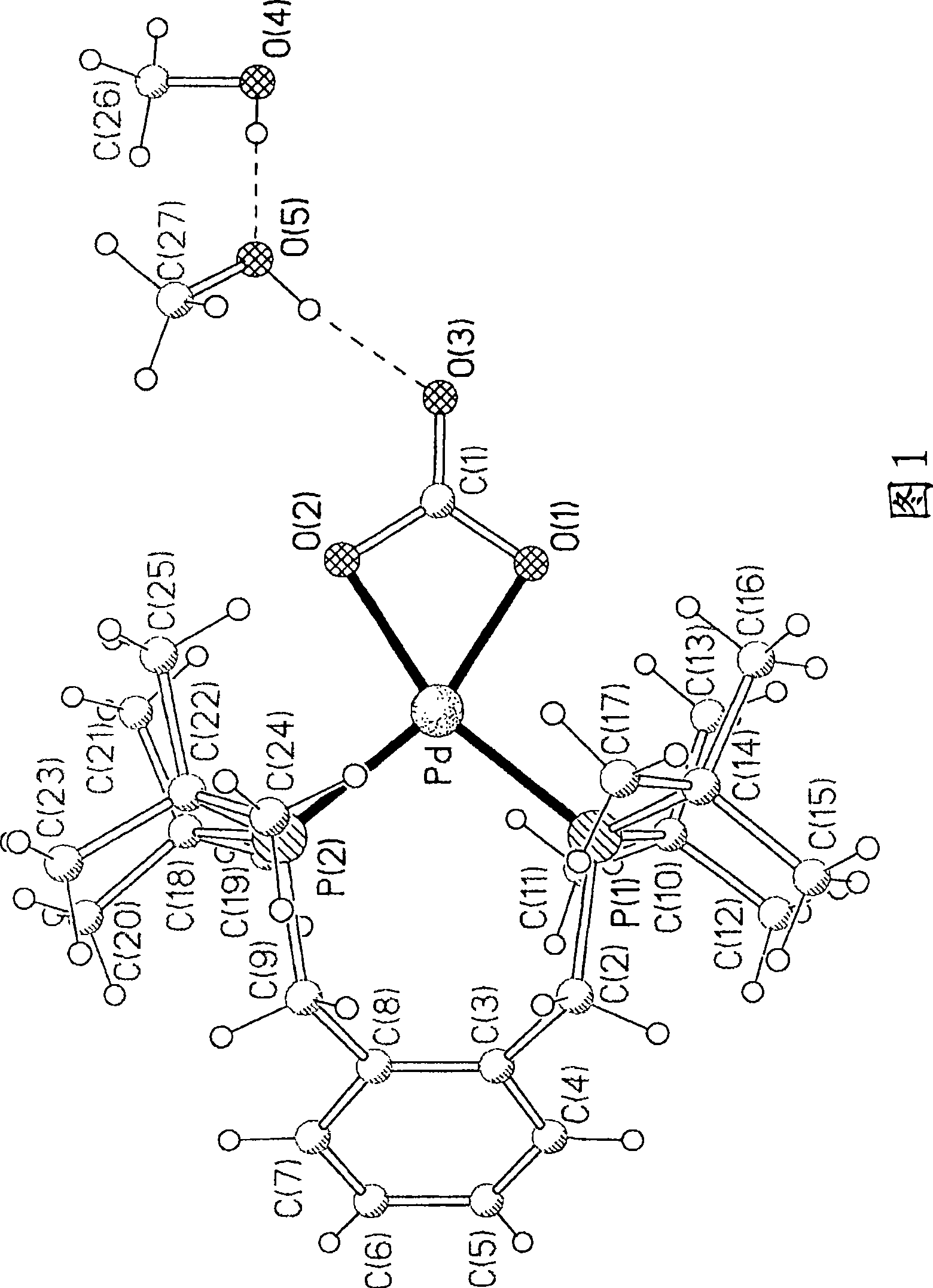
[0917] Tetraamminepalladium bis(bicarbonate) and 1 molar equivalent of 1,2-bis(di-tert-butylphosphinomethyl)benzene were suspended in methanol and refluxed for 6 hours. During the reaction, a basic gas (thought to be ammonia) evolved. The product was filtered off at room temperature and dried. The product was recrystallized from hot methanol, and the crystals were characterized by: single crystal X-ray diffraction. The product was determined to be Pd(1,2-bis(di-tert-butylphosphinomethyl)benzene)(CO 3 ).2CH 3 OH (see Figure 1). By standard wet chemical analysis, the dried product contained 18.82% Pd, which is related to the compound Pd(1,2-bis(di-tert-butylphosphinomethyl)benzene)(CO 3 ) identification is consistent. Further supportive identification was also obtained using infrared spectroscopy.
Embodiment 2
[0919] Tetraamminepalladium bis(bicarbonate) and 1 molar equivalent of 1,2-bis(di-tert-butylphosphinomethyl)ferrocene were suspended in methanol and refluxed for 6 hours. During the reaction, a basic gas (thought to be ammonia) evolved. The product was filtered off at room temperature and dried. By standard wet chemical analysis, the dried product contained 15.95% Pd, which is compatible with the compound Pd (1,2-bis(di-tert-butylphosphinomethyl)ferrocene) (CO 3 ) identification is consistent. Further supportive identification was also obtained using infrared spectroscopy.
Embodiment 3
[0921] Suspend tetraamminepalladium bis(bicarbonate) and 1 molar equivalent of 1,2-bis(di-3,5-dimethyladamantylphosphinomethyl)ferrocene in methanol and reflux for 6 hours . During the reaction, a basic gas (thought to be ammonia) evolved. The product was filtered off at room temperature and dried. By standard wet chemical analysis, the dry product contained 9.60% Pd, which is compatible with the compound Pd (1,2-bis((di-3,5-dimethyladamantyl)phosphinomethyl)ferrocene) (CO 3 ) identification is consistent. Further supportive identification was also obtained using infrared spectroscopy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com