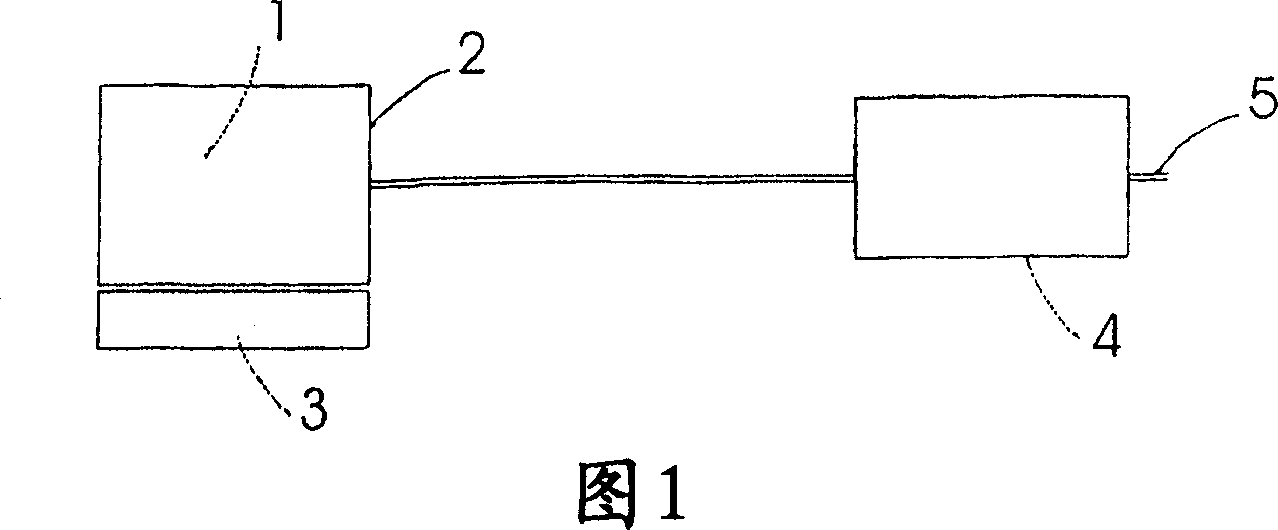
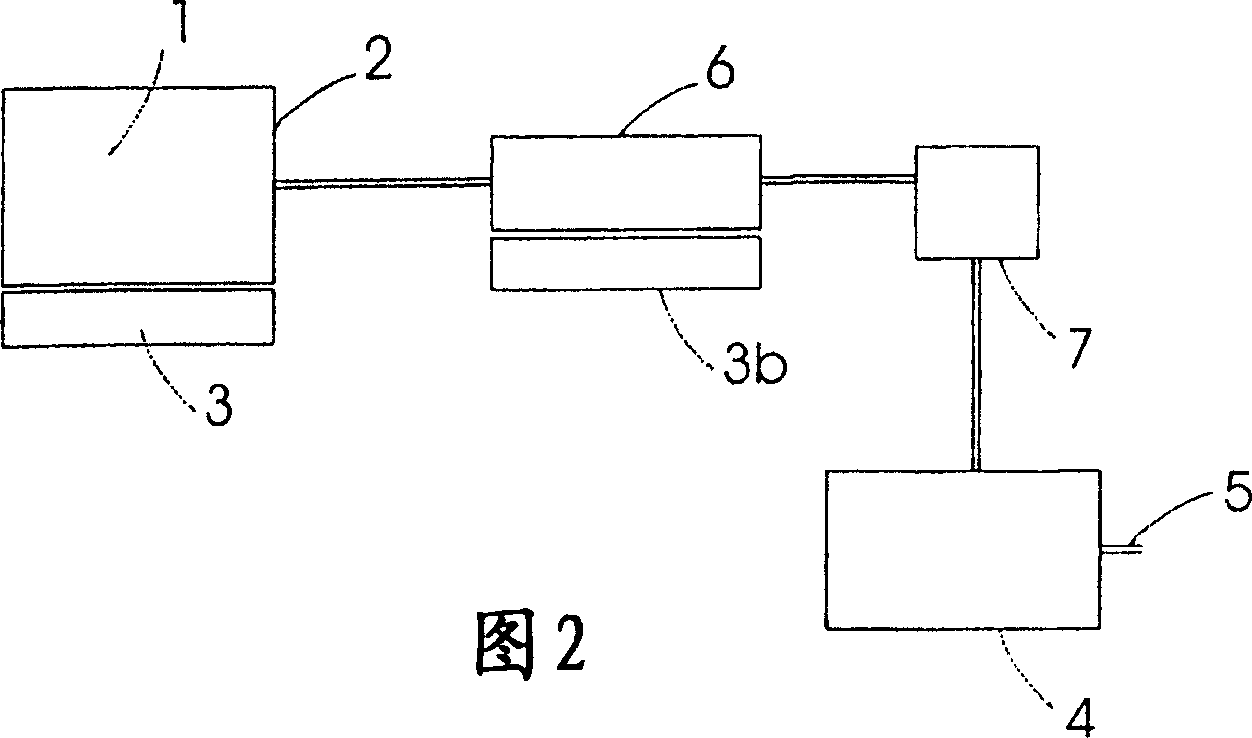
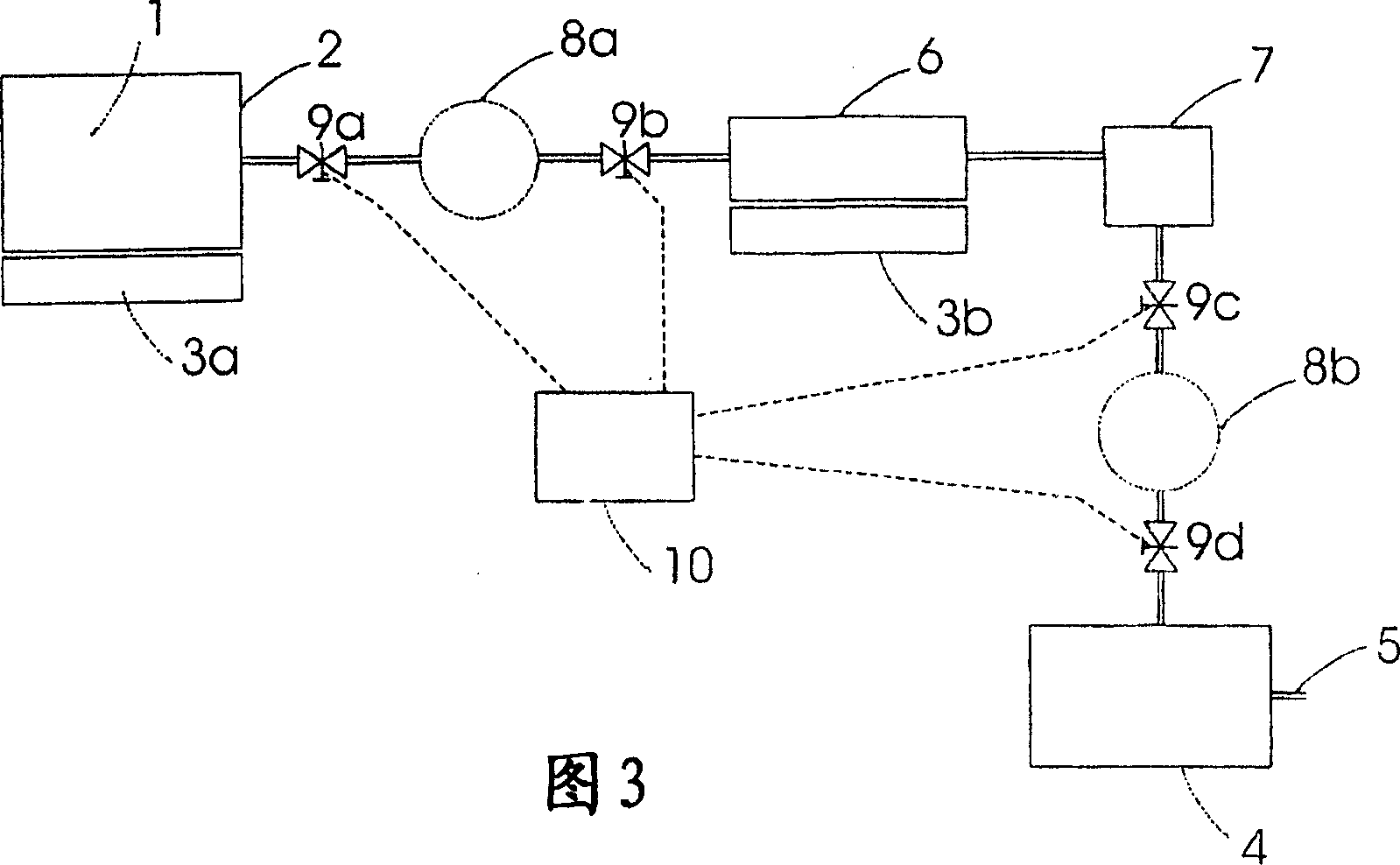
Use of an ammonia storage device in production of energy
A kind of ammonia storage and combustion device technology, which is applied in the direction of ammonia preparation/separation, ammonia treatment/storage, electrochemical generator, etc., and can solve the problems of blocking and difficult transportation of ammonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Ammonia desorption from metal halide ammines
[0096] With Ni(NH 3 ) 6 Cl 2 and Mg(NH 3 ) 6 Cl 2 To illustrate the desorption of ammonia.
[0097] From Ni(NH 3 ) 6 Cl 2 The release of ammonia in the medium is carried out as follows: 1.1g of Ni(NH 3 ) 6 Cl 2 Place in a 10 ml internal volume container equipped with a single outlet tube connected to a flow measuring device. The container was sealed and heated using electrical resistance heating at a ramp rate of 0.2°C / min. The resulting ammonia fluxes are shown in panel A, and the cumulative released amounts constitute the 2 6 moles of NH 3 The total theoretical quantity (within measurement error).
[0098] Using Mg(NH 3 ) 6 Cl 2 A similar experiment was performed to illustrate desorption from alkaline earth metal halide ammines. The resulting ammonia flux is shown in panel B, and the cumulative released amount still constitutes 2 6 moles of NH 3 The total theoretical quantity (within measur...
Embodiment 2
[0099] Embodiment 2: in general formula M a x z Acquisition of gaseous ammonia from metal salts of
[0100] The general formula is M a x z The salt was exposed to gaseous ammonia and the absorption was studied using X-ray diffraction. Study of ZnCl after exposure to gaseous ammonia at 1 bar pressure and ambient temperature 2 、CuSO 4 、CoCl 3 , MgCl 2 and NiCl 2 . The results are shown in panels C-G. An additional experiment showed that in less than 20 minutes at 8 bar ammonia pressure, MgCl 2 The ammonia absorption is complete.
Embodiment 3
[0101] Example 3: Reversibility of absorption-desorption cycle
[0102] After absorption and subsequent desorption (panel H) and after a second absorption (panel I), by MgCl 2 X-ray diffraction study of the reversibility of the absorption cycle. When compared with Figure F, it can be found that the change of the crystal structure is reversible during the absorption / desorption of ammonia.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com