Synthetic method of perfluoroisobutyronitrile
A technology of perfluoroisobutyronitrile and a synthesis method is applied in the field of synthesis of perfluoroisobutyronitrile, and can solve problems such as increased production cost, environmental pollution, long route and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
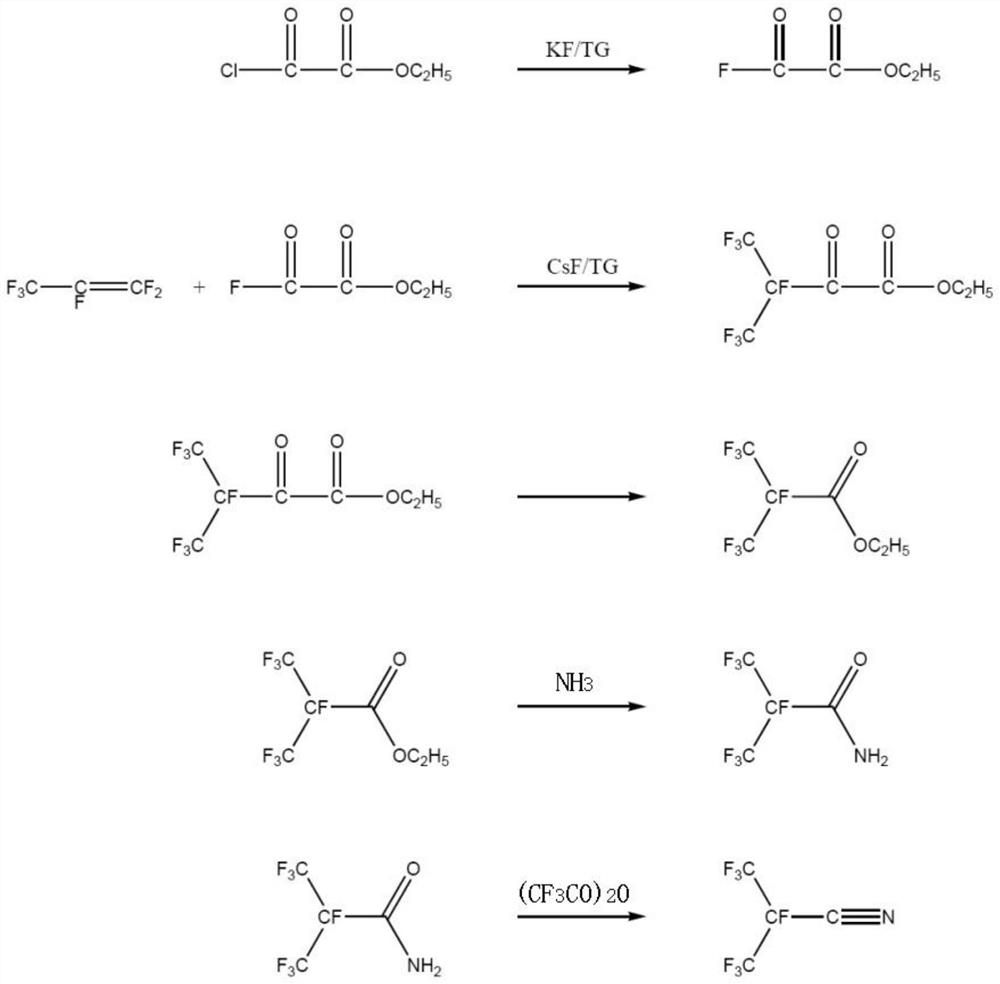
[0060] A kind of synthetic method of the perfluoroisobutyronitrile of the embodiment of the present invention, its synthetic scheme is as follows figure 1 Shown, this synthesis method comprises the following steps:
[0061] (1) Synthesis of oxalyl fluoride monoester
[0062] First, the reaction system is heated and vacuumized to remove water, and the whole system is purged with high-purity nitrogen to remove oxygen therein; the reactor is cooled to normal temperature, and under the protection of nitrogen, tetraethylene glycol dimethyl ether (444.6g) is passed into the reactor. , 2mol), add dry potassium fluoride (29.0g, 0.5mol), add monoethyl oxalyl chloride (272.9g, 2mol) and stir; heat up to 130 ° C, keep the temperature for 5 hours; separate the fluorocarbon layer after the reaction , Distilled under reduced pressure to obtain monoethyl oxalyl fluoride (232.1 g, 1.94 mol) with a yield of 97%.
[0063] (2) Synthesis of ethyl heptafluoro-2-carbonyl-3-methylbutyrate
[006...
Embodiment 2
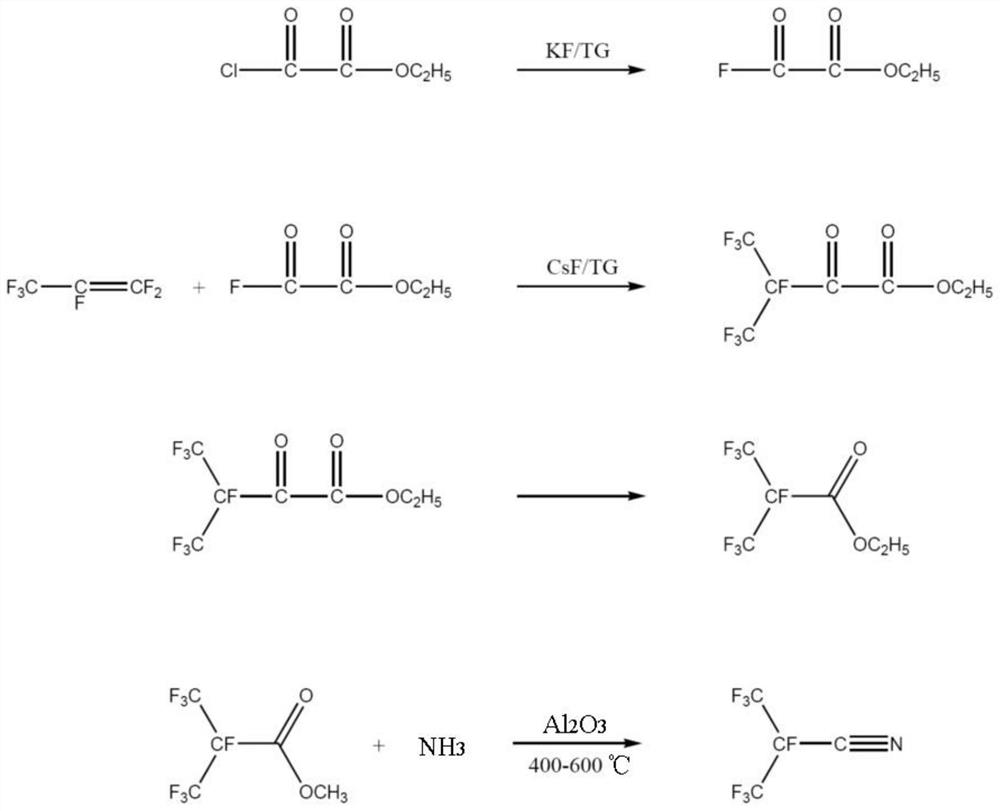
[0072] A kind of synthetic method of the perfluoroisobutyronitrile of the embodiment of the present invention, its synthetic scheme is as follows figure 2 Shown, this synthesis method comprises the following steps:
[0073] (1) Synthesis of oxalyl fluoride monoester
[0074] First, the reaction system is heated and vacuumized to remove water, and the whole system is purged with high-purity nitrogen to remove oxygen therein; the reactor is cooled to normal temperature, and under the protection of nitrogen, tetraethylene glycol dimethyl ether (444.6g) is passed into the reactor. , 2mol), add dry potassium fluoride (29.0g, 0.5mol), add monoethyl oxalyl chloride (272.9g, 2mol) and stir; heat up to 130 ° C, keep the temperature for 5 hours; separate the fluorocarbon layer after the reaction , Distilled under reduced pressure to obtain monoethyl oxalyl fluoride (232.1 g, 1.94 mol) with a yield of 97%.
[0075] (2) Synthesis of ethyl heptafluoro-2-carbonyl-3-methylbutyrate
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com