Novel biphenyl derivative as well as preparation method and medical application thereof
A pharmaceutical, phenyl technology, applied in the field of tumor treatment, biphenyl derivatives, to achieve the effect of inhibiting binding, excellent activity, and good affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
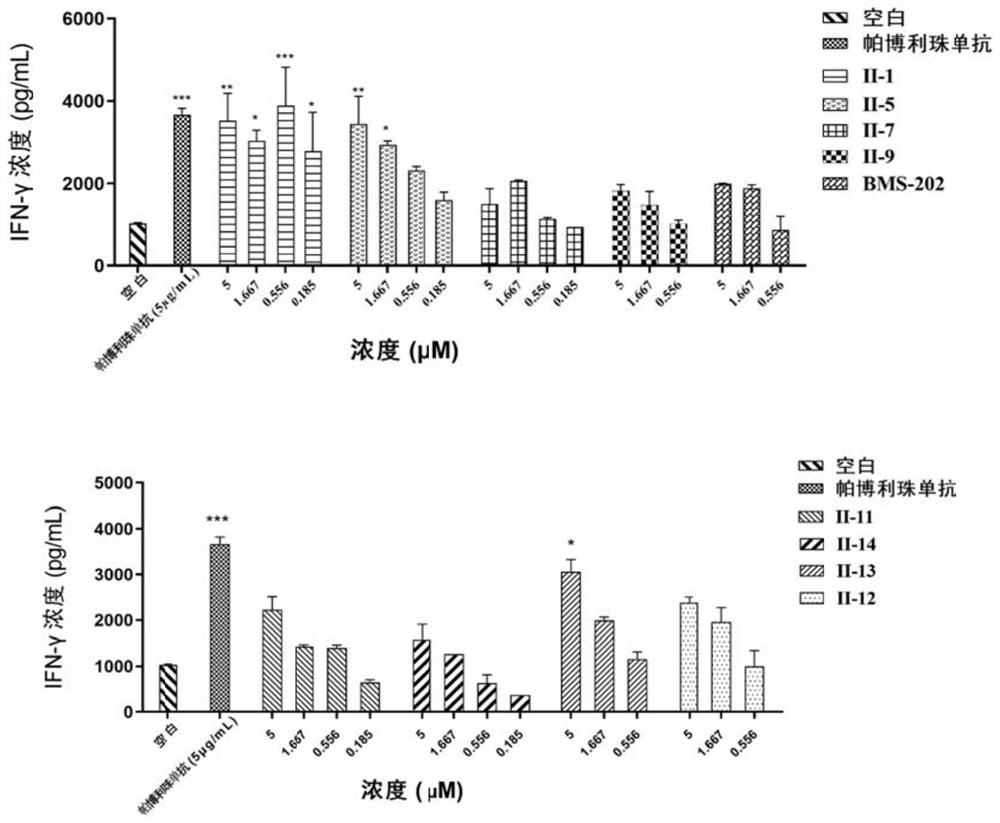
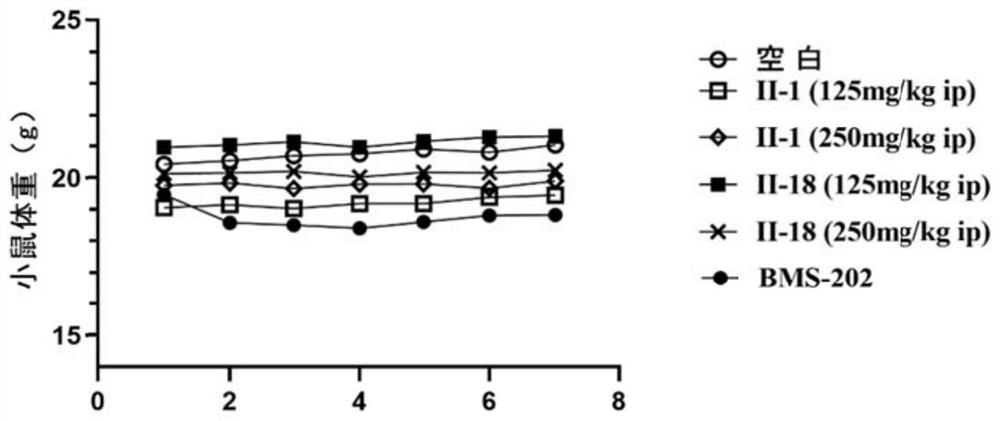
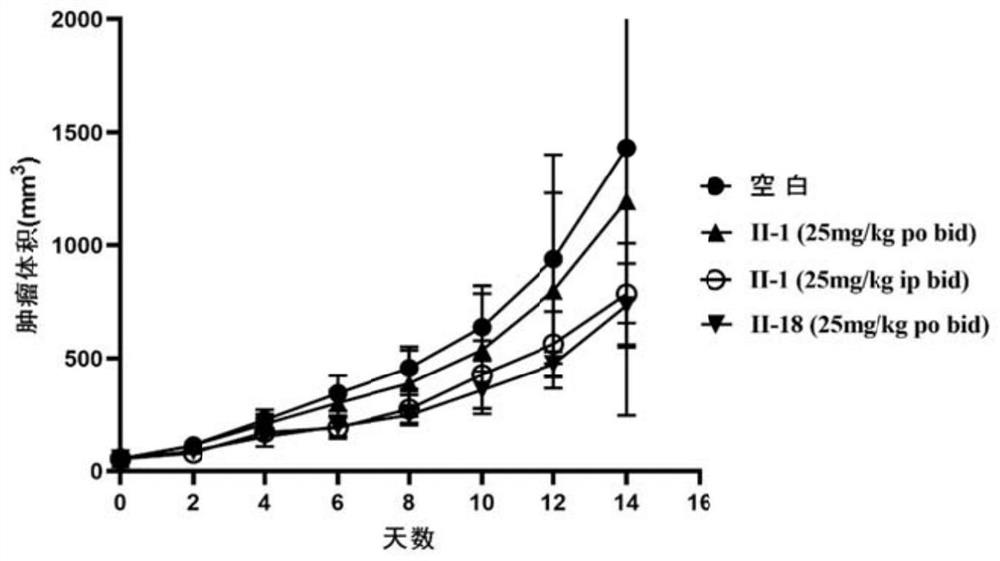
Image
Examples
Embodiment 1
[0073] (4-((3'-(3-(4-(((1-carboxy-2-hydroxyethyl)amino)methyl)-1H-1,2,3-triazol-1-yl)propane Oxy)-2,2'-dimethyl-[1,1'-biphenyl]-3-yl)methoxy)-5-chloro-2-((5-cyanopyridin-3-yl) Methoxy)benzyl)serine (II-1:R 1 and R 2 for R 3 and R 4 for -CH 3 , R 5 for Cl, R 6 for n=3) Synthesis
[0074] Synthesis of 3-bromo-2-methylphenol (IV-1)
[0075] Add 3-bromo-2-methylaniline (5.00g, 26.9mmol) into the three-necked flask, slowly drop into sulfuric acid solution (65mL, 1mmol / L) while stirring, and a large amount of insoluble white solids precipitate out. After dropping, lower the internal temperature of the reaction solution to 0-5°C, slowly add 30% sodium nitrite solution (2.23g, 32.3mmol) dropwise, yellow insoluble matter is formed, after dropping, lower the temperature to 0-5°C and stir for 30 minute. Toluene (30 mL) was added, and the temperature was raised to 100° C. to react for 1 hour. Stop the reaction after the reaction solution is clear and transparent, cool to ro...
Embodiment 2
[0113] 5-((4-chloro-2-(((2-hydroxyethyl)amino)methyl)-5-((3'-(3-(4-(((2-hydroxyethyl)amino)methyl Base)-1H-1,2,3-triazol-1-yl)propoxy)-2,2'-dimethyl-[1,1'-biphenyl]-3-yl)methoxy ) phenoxy) methyl) nicotinonitrile (II-2: R 1 and R 2 for R 3 and R 4 for -CH 3 , R 5 for Cl, R 6 for n=3) Synthesis
[0114] Dissolve compound XVI-1 (0.20g, 0.31mmol) and ethanolamine (57μL, 0.95mmol) in a mixed solution of dichloromethane (4mL) and methanol (2mL), stir at room temperature for 30min, and slowly add NaBH(OAc) 3 (0.81g, 3.80mmol), react at room temperature for 2 hours. TLC monitors that the reaction of raw materials is complete, and the reaction is stopped. Adjust the pH to 7 with saturated sodium bicarbonate solution, extract with dichloromethane (10mL×2), combine the organic phases, wash with water (10mL×2) and saturated sodium chloride solution (10mL×2), and anhydrous sodium sulfate Dry, filter with suction, and remove the solvent under reduced pressure to obtain a ligh...
Embodiment 3
[0117] N-(2-((4-((3'-(3-(4-(((2-acetylaminoethyl)amino)methyl)-1H-1,2,3-triazole-1- base)propoxy)-2,2'-dimethyl-[1,1'-biphenyl]-3-yl)methoxy)-5-chloro-2-((5-cyanopyridine-3 -yl) methoxy) benzyl) amino) ethyl) acetamide (II-3: R 1 and R 2 for R 3 and R 4 for -CH 3 , R 5 for Cl, R 6 for n=3) Synthesis
[0118] Compound XVI-1 (0.20g, 0.31mmol) and N-acetylethylenediamine (0.10g, 0.95mmol) were dissolved in dichloromethane (5mL) and added to the reaction flask successively, stirred at room temperature for 30min, and NaBH(OAc ) 3 (0.81g, 3.80mmol), react at room temperature for 2 hours. TLC monitors that the reaction of raw materials is complete, and the reaction is stopped. Adjust the pH to 7 with saturated sodium bicarbonate solution, extract with dichloromethane (10 mL×2), combine the organic phases, wash with water (10 mL×2) and saturated sodium chloride (10 mL×2), and dry over anhydrous sodium sulfate. Suction filtration, the solvent was removed under reduced pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com