Method for preparing amide
A technology of amides and organic amines, applied in the field of amides and preparation of amides, can solve the problems of harsh reaction conditions, difficult purification, low yield, etc., and achieve the effects of short reaction time, simple reaction system and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
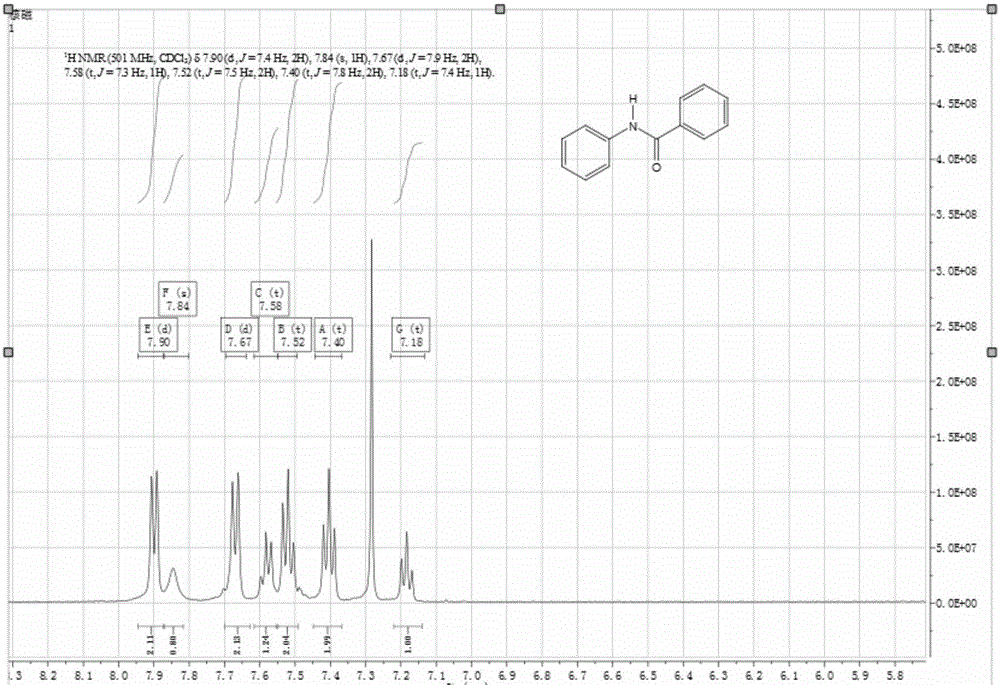
[0027] Add 1.40g of triphenoxyphosphine in a 100mL three-necked flask, and vacuum 2 , add 5mL of acetonitrile, and slowly add 0.55mL of oxalyl chloride dropwise under magnetic stirring. At this time, the reaction is violent and a large amount of gas is released, and the reaction lasts for 10 minutes. Then add 0.61g of benzoic acid and 0.59mL of aniline, and react at room temperature for 0.5 hour. The reaction is tracked by TLC, quantified by column chromatography, and qualitatively by NMR. The productive rate of benzanilide is 94%, and the NMR figure is shown in the attached figure 1 .
Embodiment 2
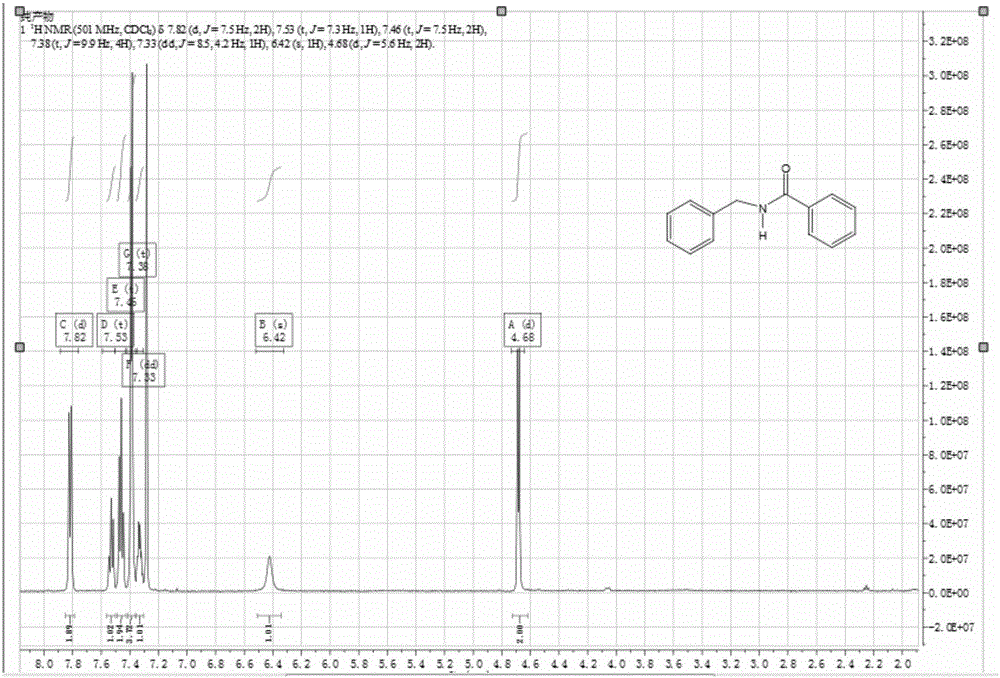
[0029] Add 1.40 g of triphenoxyphosphine into a 100 mL three-necked flask, vacuumize and ventilate with N 2 , add 5mL of acetonitrile, and slowly add 0.55mL of oxalyl chloride dropwise under magnetic stirring. At this time, the reaction is violent and a large amount of gas is released, and the reaction lasts for 10 minutes. Then add 0.61g of benzoic acid and 0.71mL of benzylamine, react at room temperature for 0.5 hour, react with TLC follow-up, carry out purification and quantification with column chromatography, the productive rate of N-benzylbenzamide is 91%, and the NMR Qualitative, see attached NMR map figure 2 .
Embodiment 3
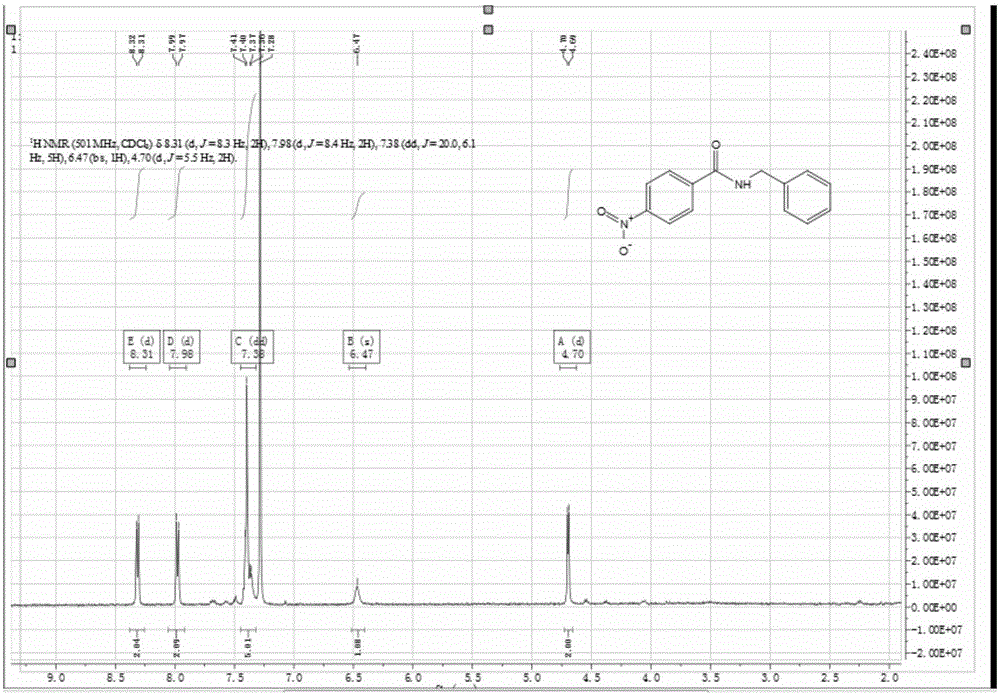
[0031] Add 1.40 g of triphenoxyphosphine into a 100 mL three-necked flask, vacuumize and ventilate with N 2 , add 5mL of acetonitrile, and slowly add 0.55mL of oxalyl chloride dropwise under magnetic stirring. At this time, the reaction is violent and a large amount of gas is released, and the reaction lasts for 10 minutes. Then add 0.86g of α-naphthoic acid and 0.71mL of benzylamine, react at room temperature for 0.5 hour, react with TLC follow-up, carry out purification and quantification with column chromatography, the productive rate of N-benzyl-α-naphthoamide is 75%, qualitative by NMR, see attached NMR image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com