Preparation method for sugammadex and intermediates thereof
A technique for sugammadex sodium and intermediates, which is applied in the field of preparation of sugammadex sodium and its intermediates, can solve the problems of low yield, difficulty in post-processing and purification, and unsuitability for industrialized large-scale production. High yield, high yield and high purity, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
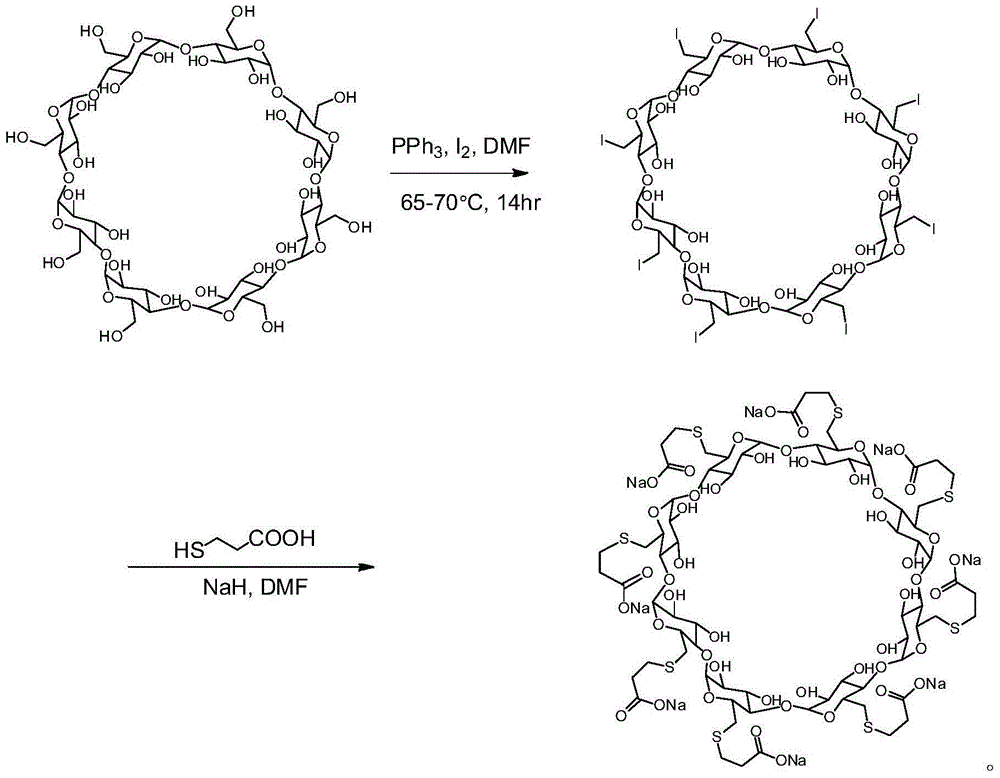
[0056] 6-perdeoxy-6-per(2-carboxyethyl)sulfide-γ-cyclodextrin (sugammadex sodium)
[0057]
[0058]
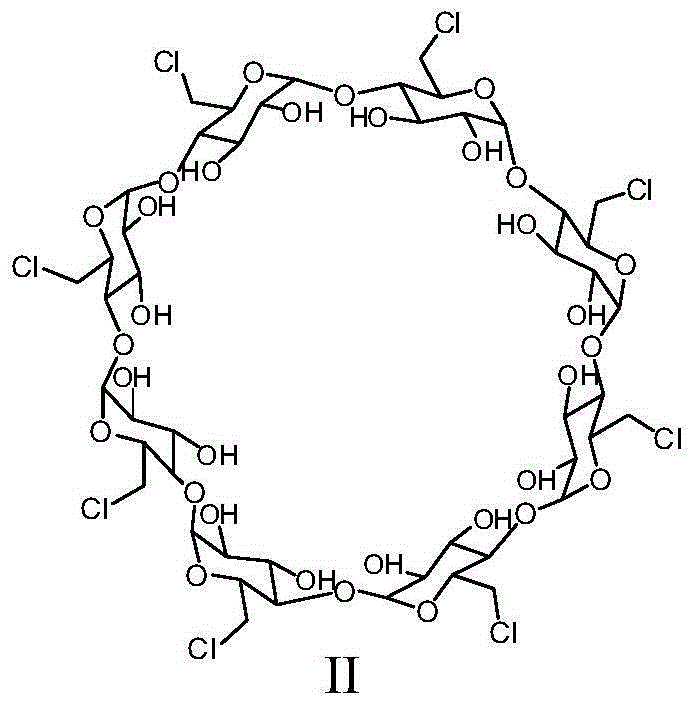
[0059] Step 1: Perchlorinated γ-cyclodextrin (compound 2)
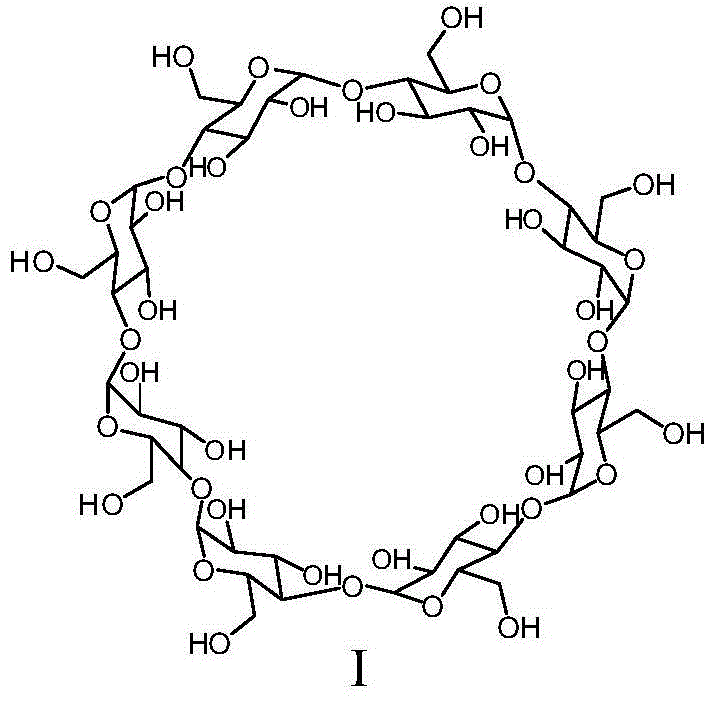
[0060] Add dry N,N-dimethylformamide (20 L) into a 50 L dry reaction kettle, replace the air with nitrogen, and cool in an ice bath. Oxalyl chloride (3.916Kg) was added dropwise at 0-5°C. During the dropwise addition, gas and solids were produced, and the reaction was violent. The dropwise addition was completed in 105 minutes, and stirring was continued for 0.5 hours. Dry γ-cyclodextrin (compound 1) (2Kg) was dissolved in dry N,N-dimethylformamide (15L), and added dropwise to the above reaction system at 5-10°C. The temperature was raised to 65-70°C, the reaction was continued for 85 hours, and the reaction was stopped. Cool the reaction solution to room temperature, add it to ice water (90L), adjust the pH value of the mixture to 8-9 with 5mol / L sodium hydroxide solution, a large amount of solids precipitat...
Embodiment 2
[0067] 6-perdeoxy-6-per(2-carboxyethyl)sulfide-γ-cyclodextrin (sugammadex sodium)
[0068]
[0069] Step 1: Perchlorinated γ-cyclodextrin (compound 2)
[0070] Add dry N,N-dimethylformamide (75 mL) into a 250 mL dry three-necked flask, replace with nitrogen, and cool in an ice bath. Thionyl chloride (29.4 g) was added dropwise at 0-5°C, and the dropwise addition was completed in 30 minutes, then the temperature was raised to room temperature and the reaction was continued with stirring for 1 hour. Add dry γ-cyclodextrin (Compound 1) (10g) into the reaction system at 5-10°C, raise the temperature to 70-75°C for 24 hours, cool the reaction solution to room temperature, and concentrate under reduced pressure to half the volume of the reaction solution , add the remaining solution to ice water (1.5 L), adjust the pH value of the mixed solution to 8-9 with sodium hydroxide, a large amount of solids are precipitated, raise the temperature of the reaction system to room temperatu...
Embodiment 3
[0074] Preparation of perchlorinated γ-cyclodextrin (compound 2)
[0075] Add dry N,N-dimethylformamide (15 L) into a 50 L dry reaction kettle, replace the air with nitrogen, and cool with an ice bath. Oxalyl chloride (3.35Kg) was added dropwise at a temperature of 0-5°C, and the addition was completed in 110 minutes, and the stirring was continued for 0.5 hour. Dry γ-cyclodextrin (compound 1) (2Kg) was dissolved in dry N,N-dimethylformamide (10L), and added dropwise to the above reaction system at 5-10°C. The temperature was raised to 65-70° C., and the reaction was continued for 20 hours. Cool the reaction solution to room temperature, add it to ice water (90L), adjust the pH value of the mixture to 8-9 with sodium hydroxide, a large amount of solids precipitate, raise the reaction temperature to room temperature and continue stirring for 1 hour, suction filtration, filter cake It was washed with water (15 L) and dried to obtain perchlorinated γ-cyclodextrin (compound 2) (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com