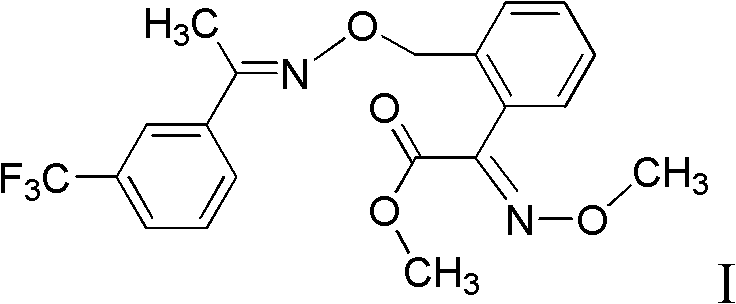
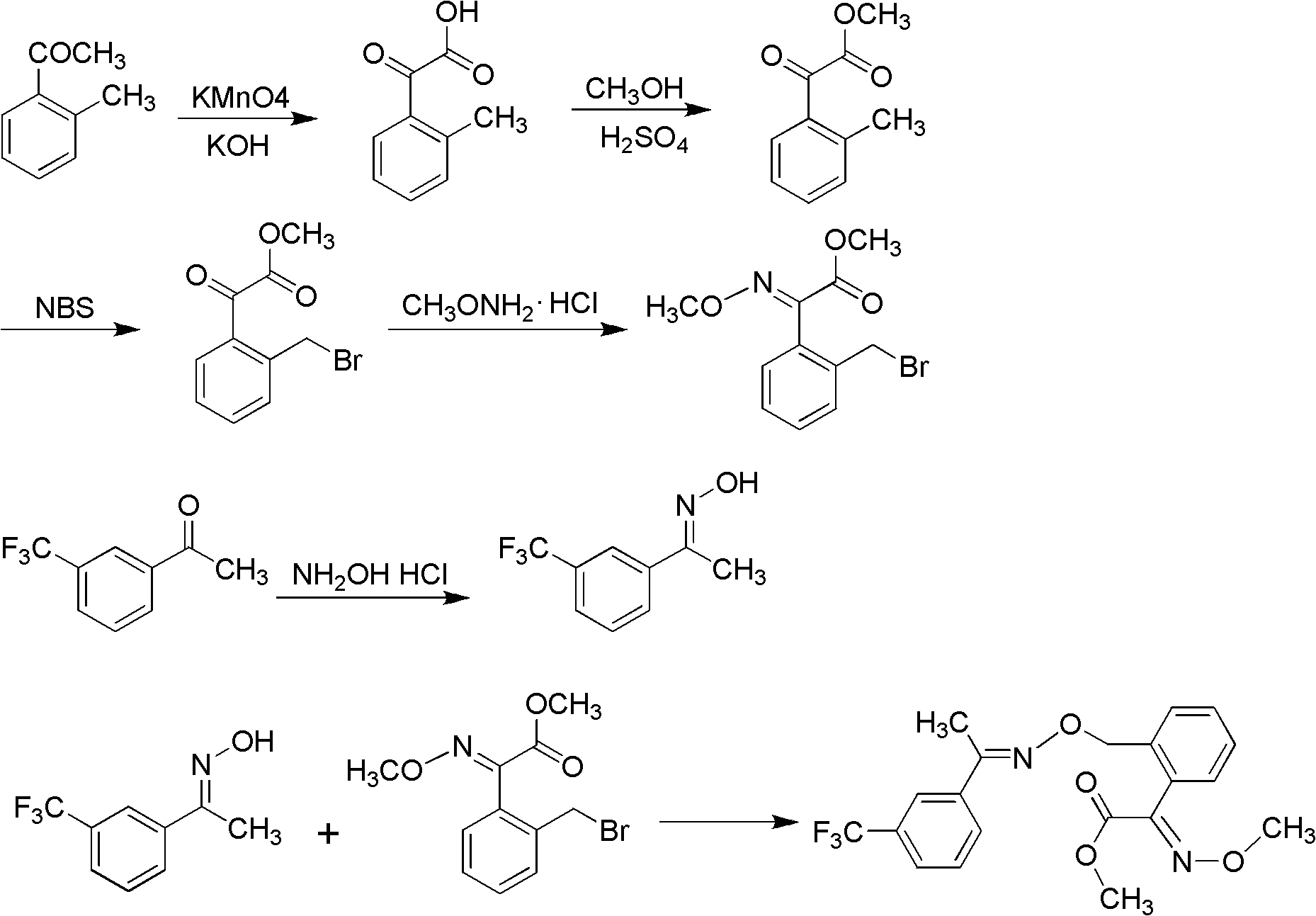
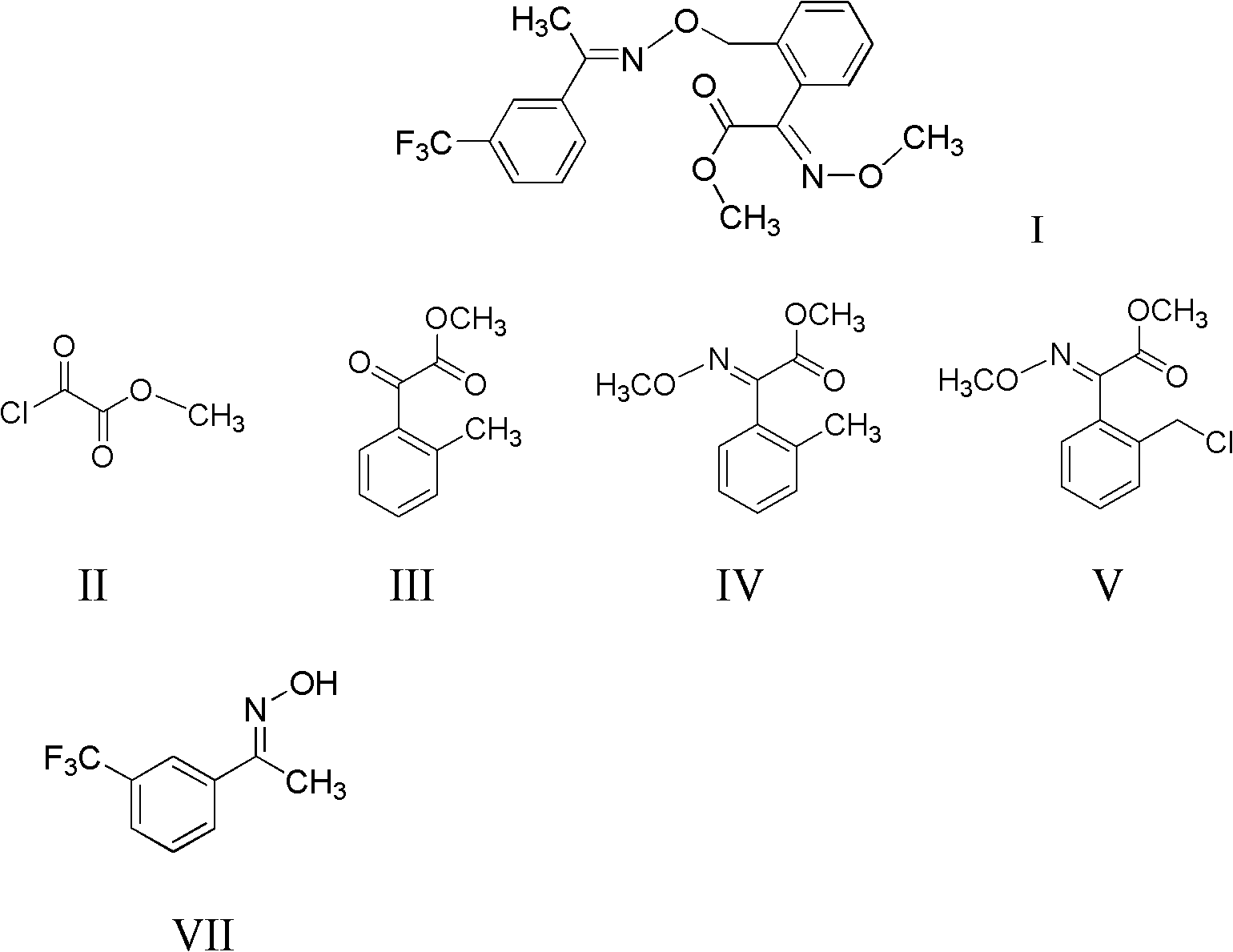
Method for preparing trifloxystrobin
A technology of trifloxystrobin and methyl ketoxime, which is applied in the field of trifloxystrobin preparation and can solve the problems of complex process and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Synthesis of 2-(2'-methylphenyl)-2-oxomethyl acetate
[0051] Dissolve 9.2g (0.1mol, percent) of toluene and 20.0g (0.15mol, percent) of anhydrous aluminum trichloride in 46g of dichloroethane, cool to 5°C with an ice bath, and slowly add oxalyl chloride mono Methyl ester 14.7g (0.12mol, percent), react at room temperature after completion of the dropwise addition, until the point of raw material toluene disappears with TLC monitoring, after the completion of the reaction, the reaction solution is slowly poured into ice water, and after standing for stratification, The organic layer was washed with water, dried with anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 16.9 g of methyl 2-(2'-methylphenyl)-2-oxoacetate, whose purity was detected by high performance liquid chromatography. 96.5%, yield 91.6%.
[0052] You can make a few more pots according to the above method, as the raw material for the next reaction.
[005...
Embodiment 2
[0065] (1) Synthesis of 2-(2'-methylphenyl)-2-oxomethyl acetate
[0066] Change the amount of anhydrous aluminum trichloride into 13.3g, change the solvent into 92g nitrobenzene, change the amount of monomethyl oxalyl chloride into 18.4g, TLC tracking detects to react completely, other conditions are the same as embodiment 1, obtain 2 -(2'-methylphenyl)-2-oxomethyl acetate 15.7g, the purity detected by high performance liquid chromatography was 95.8%, and the yield was 84.5%.
[0067] You can make a few more pots according to the above method, as the raw material for the next reaction.
[0068] (2) Synthesis of (E)-2-(2'-methylphenyl)-2-methyl oxoacetate-O-methyl ketoxime
[0069] Change the amount of methoxylamine hydrochloride into 16.7g, change the solvent into 142.4g of methyl alcohol, react between 40~45°C, follow up and detect with TLC, react completely in 15 hours, other conditions are the same as embodiment 1, 16.2 g of (E)-2-(2'-methylphenyl)-2-oxoacetic acid methyl...
Embodiment 3
[0080] (1) Synthesis of 2-(2'-methylphenyl)-2-oxomethyl acetate
[0081] Change the amount of anhydrous aluminum trichloride into 26.7g, change the solvent into 46g methylene chloride, change the amount of monomethyl oxalyl chloride into 13.5g, and other conditions are the same as embodiment 1, and obtain 2-(2'-methyl 15.1 g of methyl phenyl)-2-oxoacetate, the purity detected by high performance liquid chromatography was 96.9%, and the yield was 82.2%.
[0082] You can make a few more pots according to the above method, as the raw material for the next reaction.
[0083] (2) Synthesis of (E)-2-(2'-methylphenyl)-2-methyl oxoacetate-O-methyl ketoxime
[0084] Change the amount of methoxylamine hydrochloride into 20.8g, change the solvent into 267.4g butanol, react between 95~100°C, follow up and detect with TLC, react completely in 5 hours, other conditions are the same as embodiment 1, 15.8 g of (E)-2-(2'-methylphenyl)-2-oxoacetic acid methyl ester-O-methyl ketoxime was obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com