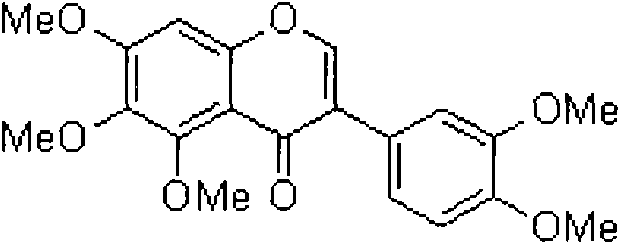
Preparation method of COMT inhibitor 5, 6, 7, 3', 4'-pentamethoxyl isoflavone
The technology of pentamethoxyisoflavone and inhibitor is applied in the field of chemical preparation, and achieves the effects of easy availability of raw materials, mild reaction conditions and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
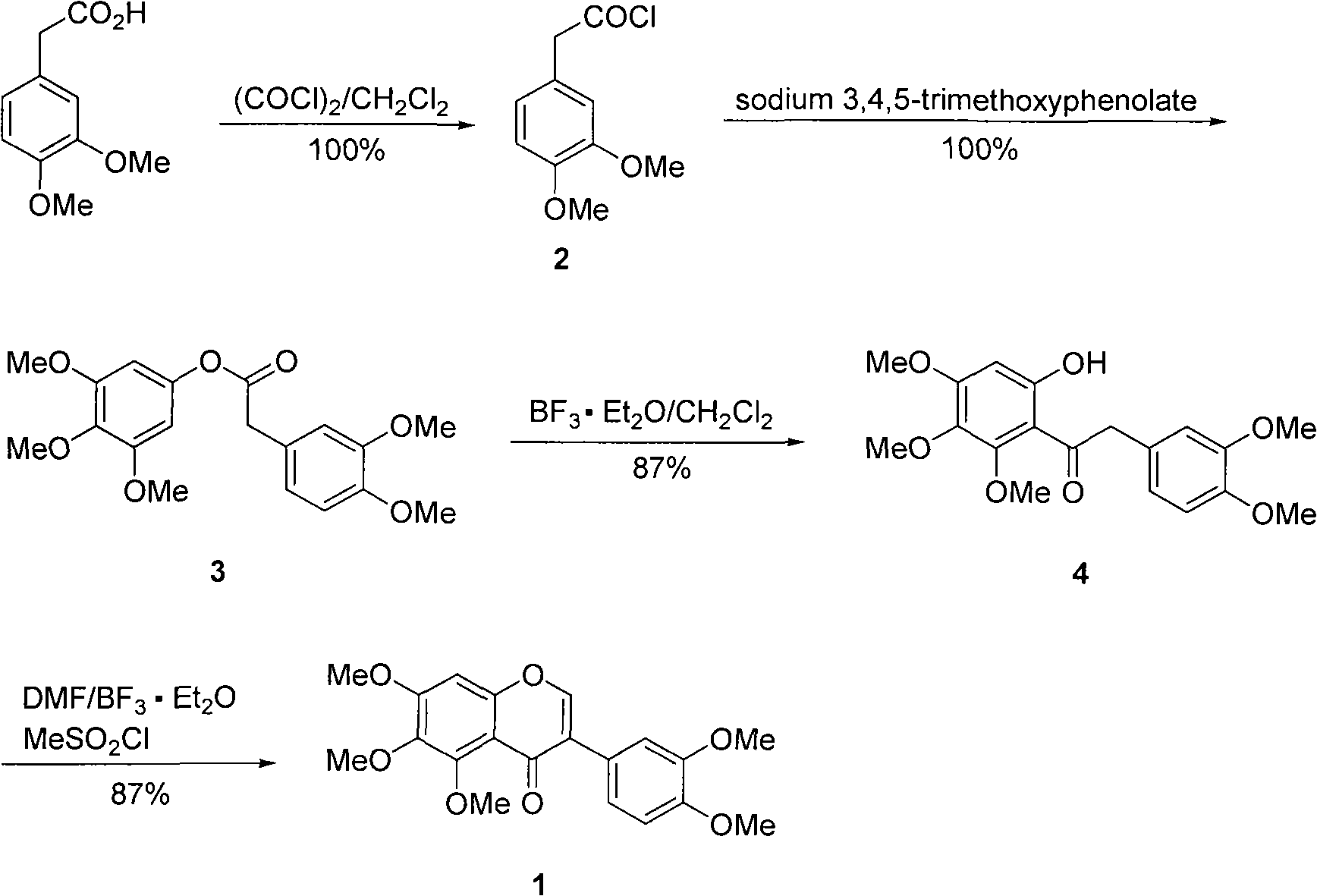
[0023] (1) Synthesis of 3,4-dimethoxyphenylacetyl chloride 2
[0024] Oxalyl chloride (5.8 mL, 11.6 mmol) was added dropwise to a solution of 3,4-dimethoxyphenylacetic acid (1.53 g, 7.8 mmol) in dichloromethane (2.0 mol / L, 10 mL) under the protection of argon, After the dropwise addition, heat the reaction mixture to reflux at 40-60° C. for 2 h, and cool to room temperature. The solvent and excess oxalyl chloride were removed under reduced pressure to obtain the product 3,4-dimethoxyphenylacetyl chloride 2, 1.67 g of a brown oil, with a yield of 100%. The product was directly used in the next reaction.
[0025] (2) Synthesis of 3,4-dimethoxyphenylacetic acid 3',4',5'-trimethoxyphenyl ester 3
[0026] Sodium hydride (dispersed in mineral oil, content 60%, 0.42g, 10.5mmol) was added to a solution of 3,4,5-trimethoxyphenol (1.20g, 6.5mmol) in tetrahydrofuran (10mL) under argon atmosphere The reaction mixture was stirred for 30 min under protection. Add compound 2 (1.67 g, 7.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com