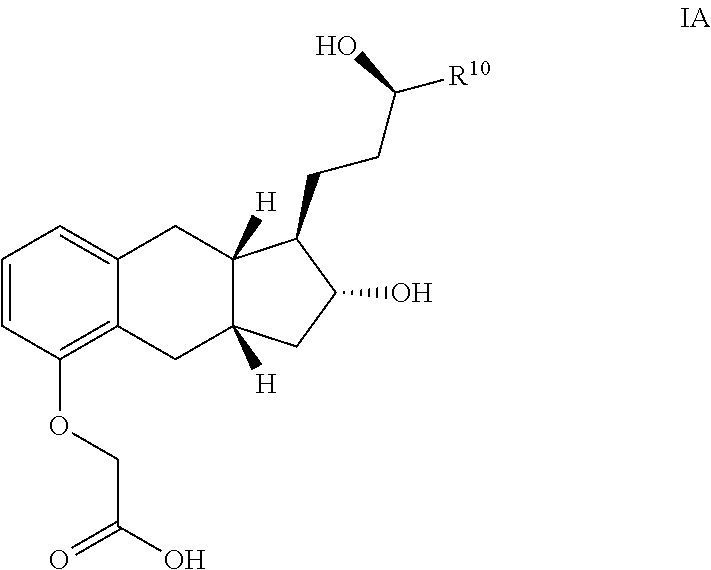
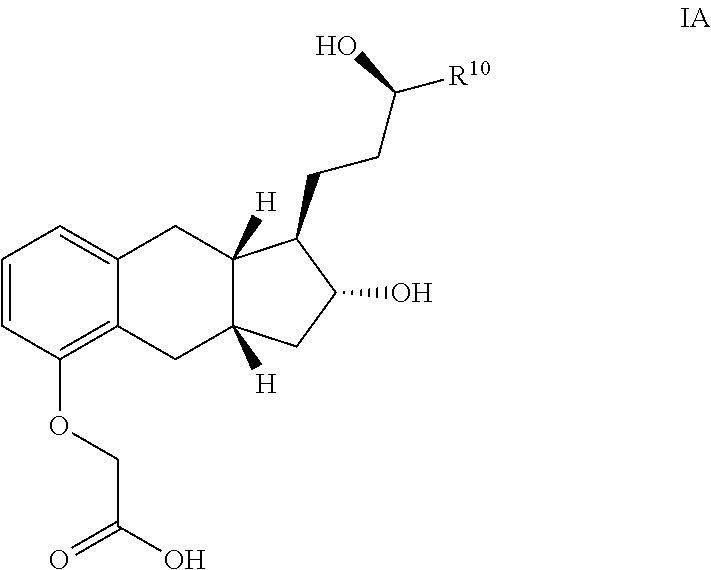
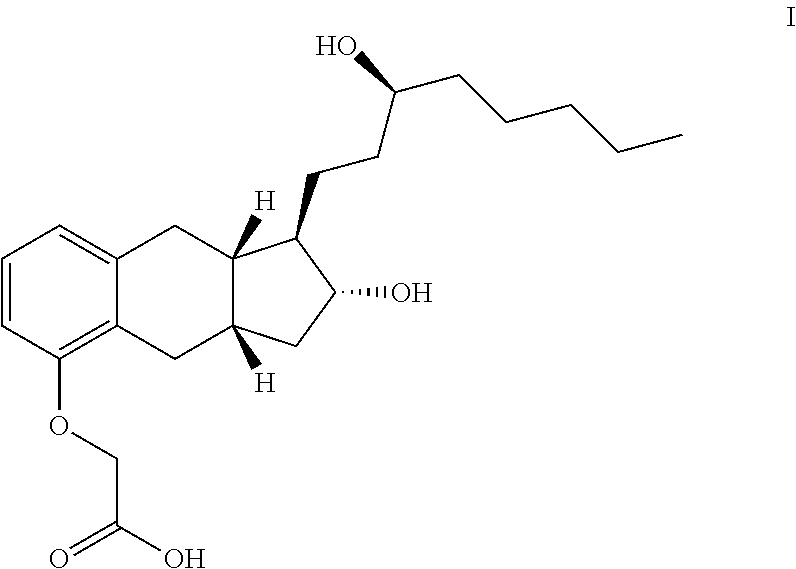
Methods of Synthesizing a Prostacyclin Analog
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(R)-oxiran-2-ylmethyl 3,5-dinitrobenzoate (1a)
[0419]
[0420]Triethylamine (8.52 g mL, 84.2 mmol, 1.25 equiv) and 4-dimethylaminopyridine (100 mg, 0.818 mmol, 0.01 equiv) were added to a solution of (S)-(−)-glycidol 1 (5.00 g, 67.5 mmol, 1.0 equiv, 99.5% ee) in anhydrous methylene chloride (100 mL) while stirring under nitrogen. The reaction was then warmed to 30° C. and 3,5-dinitrobenzoyl chloride (16.3 g, 70.9 mmol, 1.05 equiv) added drop-wise over 20 minutes as a solution in anhydrous methylene chloride (50 mL). After stirring at this temperature for 30 minutes, the reaction was quenched with addition of 10% aqueous potassium bicarbonate (50 mL) and cooled to room temperature while stirring for an additional 30 minutes. The two phases were separated and the organic phase washed with 10% aqueous citric acid (50 mL). The organic phase was then purified by filtration through a plug of silica gel giving 14.69 g of a white solid that was shown to be 99.4% e.e. by chiral HPLC. Recrystalli...
example 2
(S)-(−)-glycidol (1, ˜100% ee)
[0421]
[0422]A solution of dinitrobenzoate 1a (30.06 g, 112.1 mmol, 1.0 equiv) in anhydrous methanol (190 mL) was heated to reflux for 2 hours while stirring, under nitrogen. The reaction was then cooled to 0° C. in an ice bath causing formation of a crystalline solid that was removed by filtration and rinsed with ice cold methanol (15 mL). The filtrate was concentrated under reduced pressure resulting in formation of a white slurry that was dissolved in tert-butyl methyl ether (20 mL) and concentrated to dryness. The residue was again slurried in methanol (15 mL), the solid removed by filtration and rinsed with more methanol (5 mL). The filtrate was concentrated to give 7.6 g (92%) of the title compound as a pale yellow oil. Data for 1: Rf=0.12 (20% EtOAc / heptane).
example 3
(R)-tert-butyldimethyl(oxiran-2-ylmethoxy)silane (2a)
[0423]
[0424]To a 0° C. solution of tert-butyl(chloro)dimethylsilane (26.540 g, 176.21 mmol, 1.3 equiv) and imidazole (14.786 g, 217.19 mmol, 1.6 equiv) in dimethylformamide (80 mL) was added (S)-oxiran-2-yl methanol (10.013 g, 135.16 mmol, 1.0 equiv) drop-wise and the resulting mixture stirred at that temperature under nitrogen for 30 minutes. The reaction was then quenched with addition of saturated aqueous ammonium chloride (200 mL) and water (200 mL). The resulting mixture was extracted with heptane (5×200 mL) and the combined organic phases were washed with brine, dried (MgSO4) and concentrated to give 25.142 g (99%) of the title compound as a yellow oil. This material was used in the next step without purification. Data for 2a: Rf=0.64 (20% EtOAc / heptane); 1H NMR (400 MHz, CDCl3) δ 3.85 (dd, J=3.22, 12.01 Hz, 1H), 3.66 (dd, J=4.69, 12.01 Hz, 1H), 3.05-3.12 (m, 1H), 2.76 (dd, J=4.25, 5.13 Hz, 1H), 2.63 (dd, J=2.64, 4.98 Hz, 1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com