Synthesis method for N-Boc-3-piperidone
A technology of n-boc-3- and 1.n-boc-3-, which is applied in the field of pharmaceutical intermediate synthesis, can solve problems such as excessively long synthetic route of N-Boc-3-piperidone, and achieve short synthetic route. , the effect of reducing production costs and energy consumption, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
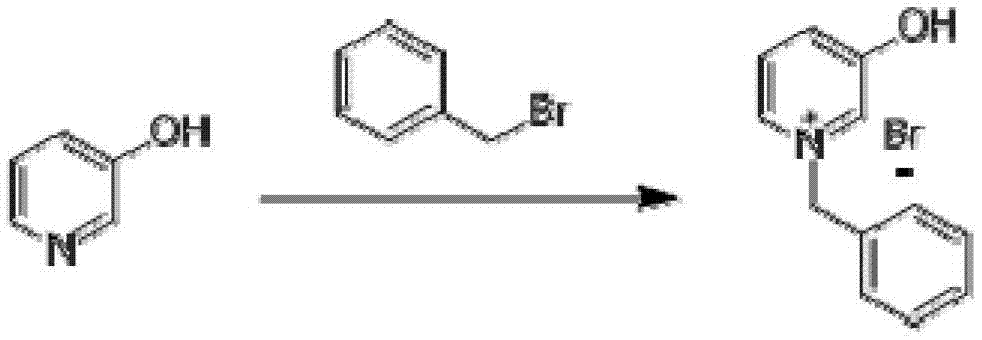
[0027] first step:
[0028]
[0029] Dissolve 95 g of 3-hydroxypyridine (1.0 mol) in 500 ml of ethanol, cool the reaction solution below 5°C, add 180 g of benzyl bromide (1.05 mol) dropwise, drop it in half an hour, then return to room temperature and stir overnight . The completion of the reaction was monitored by TLC, filtered directly, and the filter cake was washed three times with 50 ml of ethanol, and then dried to obtain N-benzyl-3-hydroxypyridine quaternary ammonium salt (240 g, 0.90 mol), with a yield of 90%.
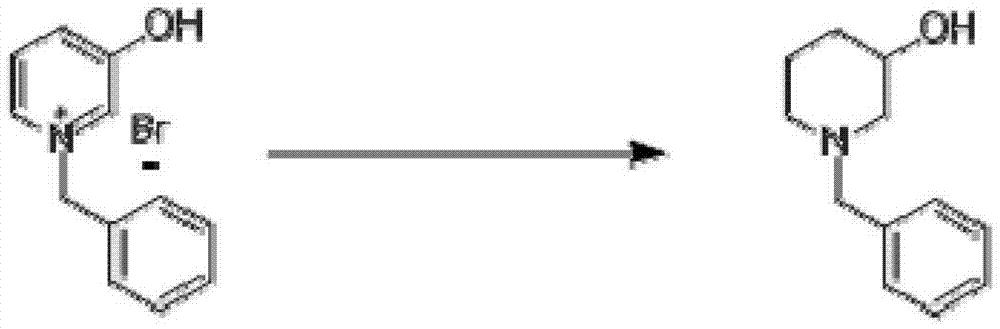
[0030] Step two:
[0031]
[0032] Dissolve 266 grams of N-benzyl-3-hydroxypyridine quaternary ammonium salt (1.0mol) in 1500 milliliters of ethanol, cool down to about 0°C in an ice-salt bath, slowly add 80 grams of sodium borohydride (2.11mol), when adding Keep the temperature at 0°C all the time. After the addition, the reaction solution returns to room temperature and reacts overnight. TLC monitors that the reaction is complete, add 100 milliliters...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com