Preparation method of lithium difluorobisoxalate phosphate solution
A technology of lithium difluorobisoxalate phosphate and oxalic acid solution, which is applied in the field of electrolyte, can solve the problems of large environmental pollution of by-products, unsuitability for industrialization, complicated preparation methods, etc., and achieve simple post-reaction treatment, increase feasibility, and reaction Effects in simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] 2. Preparation of lithium difluorobisoxalate phosphate solution
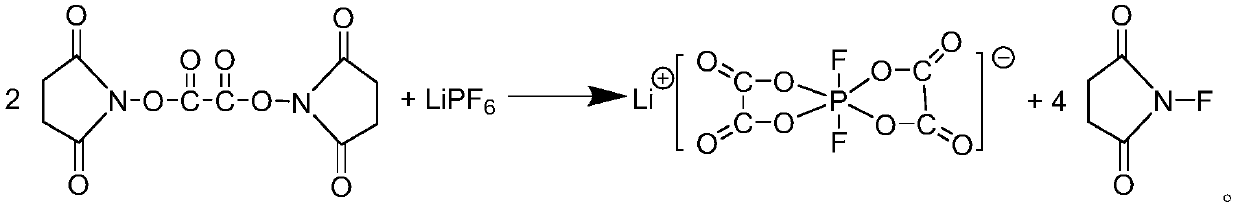
[0041] Add lithium hexafluorophosphate to the above-mentioned two (N-succinimidyl) oxalic acid solution, react, and process after the reaction to obtain difluorobisoxalate lithium phosphate solution, the reaction equation is as follows:
[0042]
[0043] In some embodiments, the molar ratio of lithium hexafluorophosphate to bis(N-succinimidyl)oxalic acid is 1:2-2.2, for example: 1:2, 1:2.1, 1:2.2.
[0044] The operation of adding lithium hexafluorophosphate needs to be carried out in an inert gas environment with a moisture content of less than 10 ppm, such as in a glove box. The temperature at the time of addition was controlled at 0°C.
[0045] The reaction temperature is 30-80° C., and the reaction time is 4-8 hours.
[0046] In some embodiments, the reaction temperature is 50-80°C, for example: 50°C, 55°C, 60°C, 65°C, 70°C, 75°C, 80°C, etc.
[0047] Non-limiting examples of the reaction time inclu...
Embodiment 1
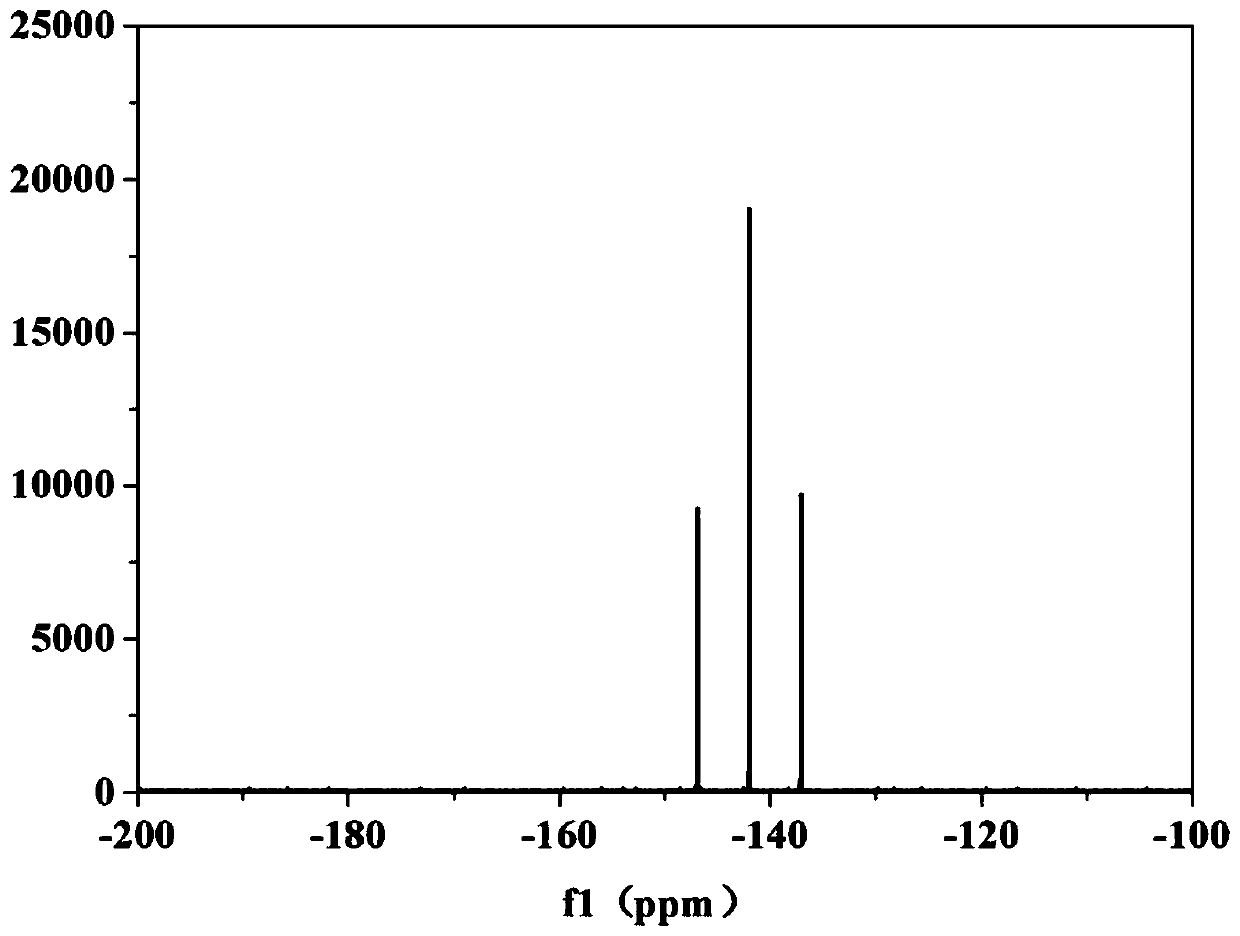
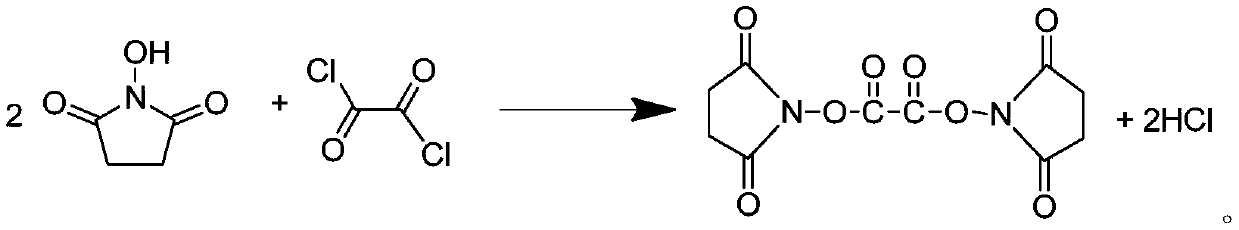
[0054] In a 500mL three-necked flask, add 200g of dimethyl carbonate, 57.5g (0.504mol) of N-hydroxysuccinimide and 63g (0.496mol) of oxalyl chloride, and react at 60°C for 9h under the protection of nitrogen to produce The tail gas can be absorbed by a low-concentration sodium hydroxide solution; after the reaction is completed, feed dry nitrogen into the reaction solution at 80°C for 5 hours to remove excess oxalyl chloride and HCl to obtain bis(N-succinimidyl)oxalic acid solution. Then, the reaction flask containing bis(N-succinimidyl) oxalic acid solution (containing 0.248mol bis(N-succinimidyl) oxalic acid) was transferred to the glove box, and 19 g (0.125 mol) Lithium hexafluorophosphate, after the lithium hexafluorophosphate is completely dissolved, transfer the flask to an oil bath at a temperature of 50°C, and react for 5 hours under the protection of nitrogen. 5-diketone, after filtration, a lithium difluorobisoxalate phosphate solution was obtained.
[0055] The ob...
Embodiment 2
[0060] In a 1000mL three-necked flask, add 500g of dimethyl carbonate, 172.6g (1.514mol) of N-hydroxysuccinimide and 126g (0.992mol) of oxalyl chloride, and react at 80°C for 9h under the protection of nitrogen to produce The tail gas can be absorbed by a low-concentration sodium hydroxide solution; after the reaction is completed, feed dry nitrogen into the reaction solution at 80°C for 5 hours to remove excess oxalyl chloride and HCl to obtain bis(N-succinimidyl)oxalic acid solution. Then, the reaction flask containing bis(N-succinimidyl) oxalic acid solution (containing 0.757mol bis(N-succinimidyl) oxalic acid) was transferred to the glove box, and 56.9 g (0.374 mol) lithium hexafluorophosphate, after the lithium hexafluorophosphate is completely dissolved, transfer the flask to an oil bath at a temperature of 60°C, and react for 5 hours under the protection of nitrogen, after the reaction, cool the reaction solution to 0°C, and precipitate solid 1-fluoropyrrolidine-2, 5-d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com