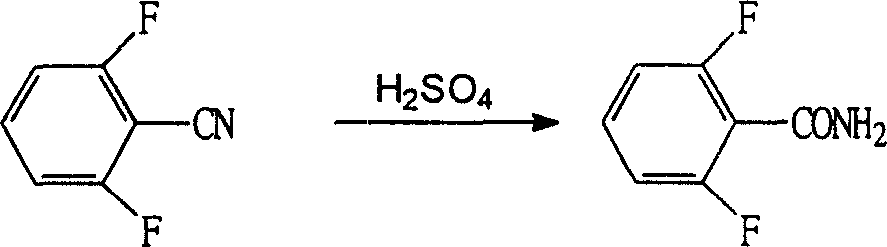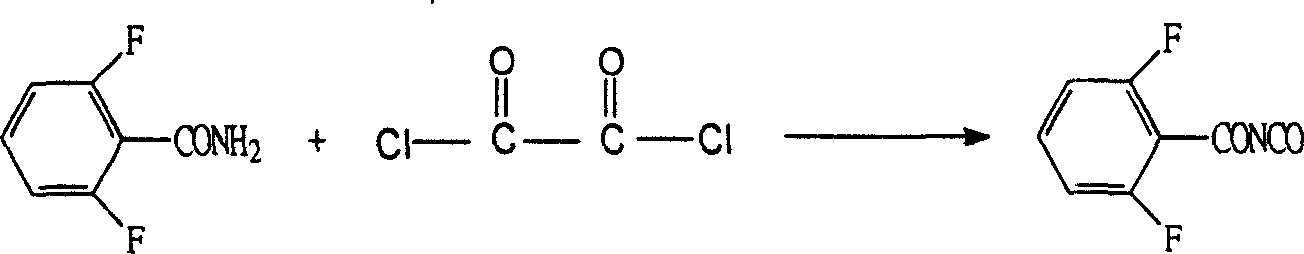Substituted benzoyl urea insect growth regulator synthesizing method
A technology for growth regulators and benzoyl ureas, which is applied in the field of synthesis of substituted benzoyl urea insect growth regulators, can solve the problems of dangerous operation, product separation, low yield and the like, and achieves easy industrialization Production, simplifying industrial operations, increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
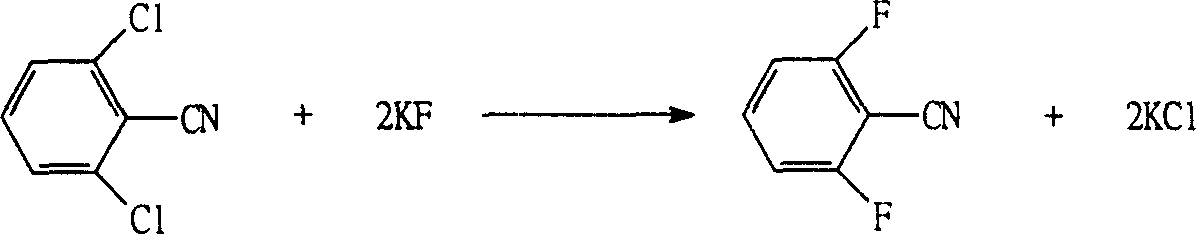
[0047] 1) Fluorination: add 500Kg of sulfolane into a 1000L reactor, add spray-dried fine-grained potassium fluoride (450 mesh) 88.4Kg (1.5KM), dichlorobenzonitrile 172Kg (1KM) while stirring, and The catalyst mixture composed of 40wt% tetrabutylammonium chloride and 60wt% methyl trioctyl ammonium chloride was 1.72Kg, heated at 200°C for 8 hours, filtered to remove the precipitated potassium chloride, and the filtrate was fractionated to obtain difluoro Benzoonitrile 133.6Kg (0.96KM), the yield was 96.1%, the content was 98.4%, and the solvent sulfolane was recovered;
[0048] 2) Hydrolysis: add 109.9Kg (0.79KM) of difluorobenzonitrile and 1.10Kg of sodium hydroxide as the catalyst into the reactor, turn on the hot steam to increase the temperature, add 72.63Kg (2.14KM) of hydrogen peroxide dropwise within 2 hours, and add dropwise After completion, keep it at 40°C for 1 hour, negative pressure excess hydrogen peroxide, and spin dry the reactants with a centrifuge to obtain a difl...
Embodiment 2
[0052]1) Fluorination: add 500Kg of sulfolane into a 1000L reactor, add spray-dried granular potassium fluoride (550 mesh) 117.8Kg (2KM), dichlorobenzonitrile 172Kg (1KM) while stirring, and A catalyst mixture composed of 60wt% tetrabutylammonium chloride and 40wt% methyl trioctyl ammonium chloride was 5.16Kg, heated at 200°C for 10 hours, filtered to remove the precipitated potassium chloride, and the filtrate was fractionated to obtain difluorobenzene Nitrile 134.1Kg (0.965KM), the yield was 96.5%, the content was 99.3%, and the solvent sulfolane was recovered;
[0053] 2) Hydrolysis: add 109.9Kg (0.79KM) of difluorobenzonitrile, catalyst sodium hydroxide 1.10Kg and sodium carbonate 1.10Kg into the reaction kettle, turn on the hot steam to increase temperature, add 51Kg (1.5KM) peroxide dropwise within 2 hours After the addition of hydrogen, keep it at 40°C for 1 hour, negative pressure excess hydrogen peroxide, and spin dry the reactants with a centrifuge to obtain a difluorobe...
Embodiment 3
[0057] 1) Fluorination: add 500Kg of sulfolane into a 1000L reactor, add spray-dried fine-grained potassium fluoride (500 mesh) 106.0Kg (1.8KM), dichlorobenzonitrile 172Kg (1KM) while stirring, and The catalyst mixture consisting of 50wt% tetrabutylammonium chloride and 50wt% methyl trioctyl ammonium chloride was 3.44Kg, heated at 200°C for 9 hours, filtered to remove the precipitated potassium chloride, and the filtrate was fractionated to obtain difluoro Benzoonitrile 133.7Kg (0.96KM), the yield was 96.2%, the content was 98.8%, and the solvent sulfolane was recovered;
[0058] 2) Hydrolysis: add 109.9Kg (0.79KM) of difluorobenzonitrile, catalyst sodium bicarbonate 1.65Kg and sodium carbonate 1.65Kg into the reaction kettle, turn on the hot steam to increase temperature, add 102Kg (3.0KM) peroxide dropwise within 2 hours After the addition of hydrogen, keep it at 40°C for 1 hour, negative pressure excess hydrogen peroxide, and spin dry the reactants with a centrifuge to obtain a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com