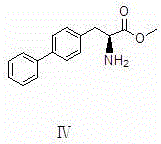
Preparation method of LCZ696 intermediate
A technology of intermediates and ligands, which is applied in the field of preparation of aromatic ring compounds, can solve the problems of unsuitability for industrial scale-up production, expensive starting raw materials, and large amount of solvents used, so as to avoid the use of heavy metal catalysts and reduce heavy metals The effect of the use of catalyst and the convenience of obtaining materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Put 2g of monomethyl oxalyl chloride and 8ml of tetrahydrofuran into the reaction flask, cool down to -10~0°C, add 15.94g of 20% benzylmagnesium bromide tetrahydrofuran solution dropwise, add dropwise for 1h, keep stirring for 2h after dropping, add 8ml of 1N hydrochloric acid, continued to stir, added 12ml of ethyl acetate to extract three times, combined the organic phases, washed with 4ml of saturated sodium chloride, and concentrated to no fraction to obtain 2.33g of the compound of formula I, with a yield of 80%.
Embodiment 2
[0038] Put 10g of monomethyl oxalyl chloride and 40ml of tetrahydrofuran into the reaction bottle, cool down to -10~0°C, add 53.1g of 20% benzylmagnesium bromide tetrahydrofuran solution dropwise, add dropwise for 1h, keep stirring for 2h after dropping, add 40ml of 1N hydrochloric acid, continued to stir, added 60ml of ethyl acetate to extract three times, combined the organic phases, washed with 20ml of saturated sodium chloride, and concentrated to no fraction to obtain 7.56g of the compound of formula I with a yield of 78%.
Embodiment 3
[0040] Put 50g of monomethyl oxalyl chloride and 200ml of tetrahydrofuran into the reaction bottle, cool down to -10~0°C, add 199.3g of 20% benzylmagnesium bromide tetrahydrofuran solution dropwise, add dropwise for 2h, keep stirring for 2h after dropping, add 200ml of 1N hydrochloric acid, continued to stir, added 300ml of ethyl acetate to extract three times, combined the organic phases, washed with 100ml of saturated sodium chloride, and concentrated to no fraction to obtain 30.15g of the compound of formula I, with a yield of 78%.
[0041]
[0042] Using the compound of formula I as the substrate, a series of bromination reagents were compared and screened. The chemical equation is as follows. The bromination reaction was carried out at 10-35°C for 4-10 hours. The results are shown in Table 1: sodium bromide, tetra-tert-butyl Ammonium tribromide, N-bromosuccinimide and bromine can be used as bromination reagents to perform bromination well.
[0043]
[0044]
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com