Fluorescence molecular probe compound as well as preparation method and application thereof
A technology for fluorescent molecular probes and compounds, applied in the field of chemical analysis and detection, can solve problems such as destroying fluorescence characteristics, and achieve the effects of excellent optical performance, increased fluorescence intensity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of 1,2-bis(4-bromo-phenyl)-1,2-diphenylethylene:
[0048] Add 4-bromo-benzophenone (8.0g, 30.8mmol), Zn powder (7.8g, 120mmol) into 250mL anhydrous tetrahydrofuran, add titanium tetrachloride (6mL, 53mmol) dropwise under ice-cooling, and then heat to reflux 8h. The reaction process was monitored by thin-layer chromatography. After the reaction of the raw materials was completed, the reaction mixture was cooled to room temperature, and the solvent was distilled off. Then it was extracted with dichloromethane, and the organic phase was washed with saturated brine and water, respectively. Separate the organic phase, add anhydrous sodium sulfate to dry, distill off the solvent, and separate and purify by column chromatography (developing agent is petroleum ether, R f =0.6) to obtain 1,2-bis(4-bromo-phenyl)-1,2-diphenylethylene as a pure white solid, with a yield of 5.21 g and a yield of 69.4%.
[0049] h 1 NMR (400MHz, CDCl 3 ):δ ppm =7.30(1H,s),7.28(1H,s)...
Embodiment 2
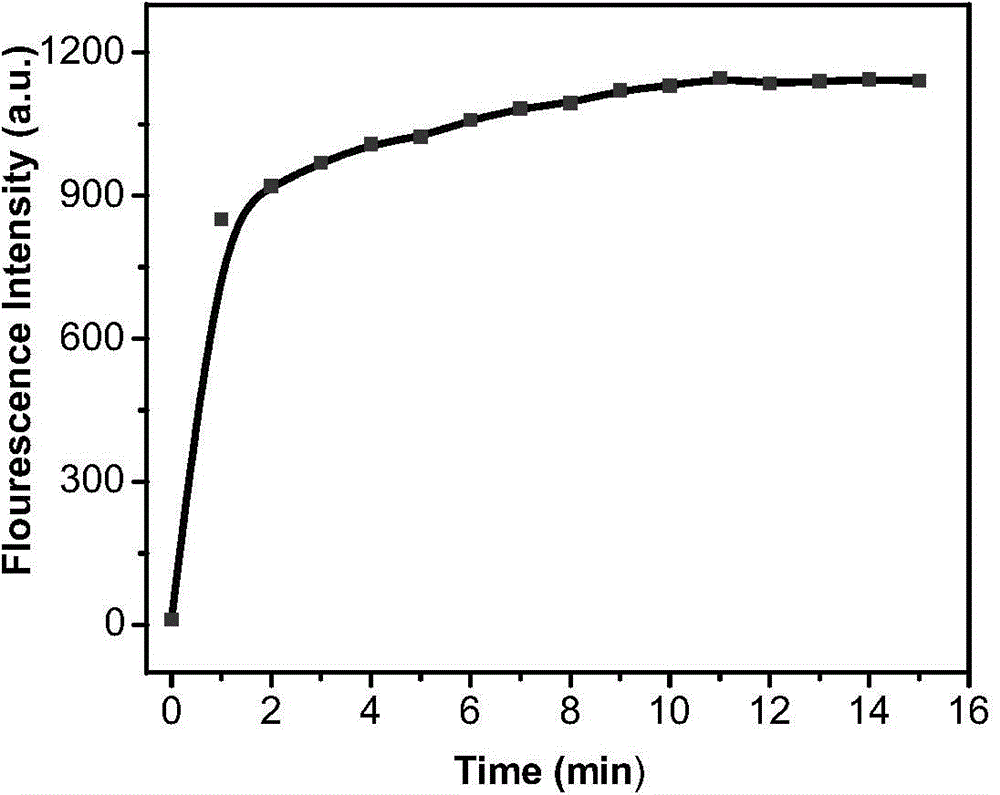
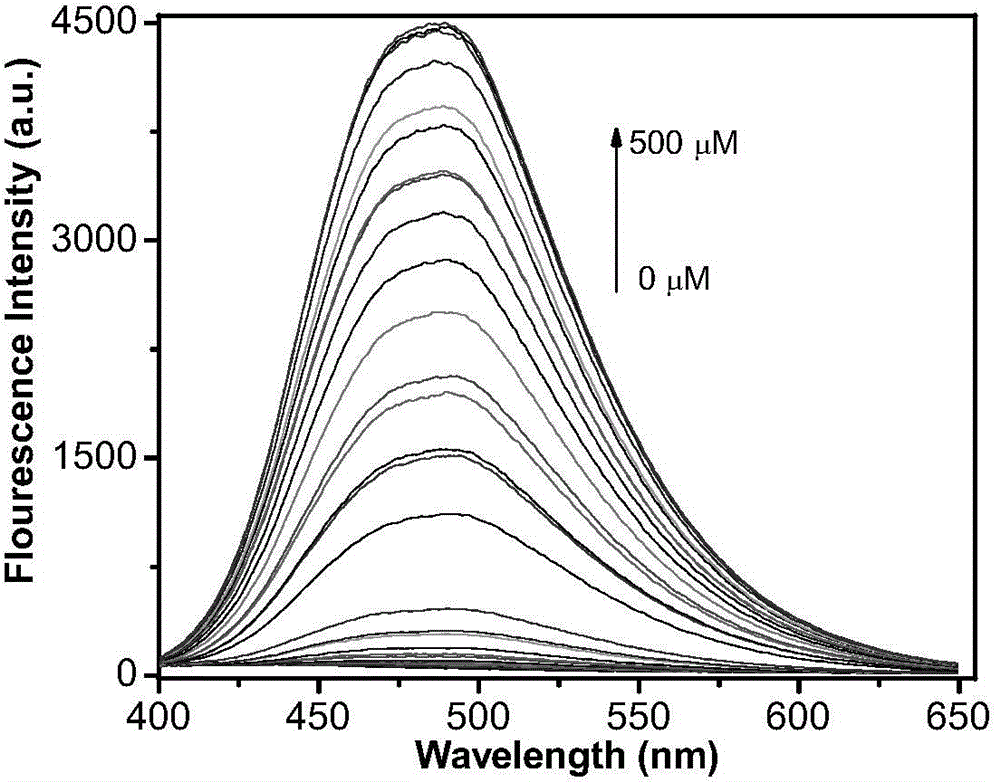
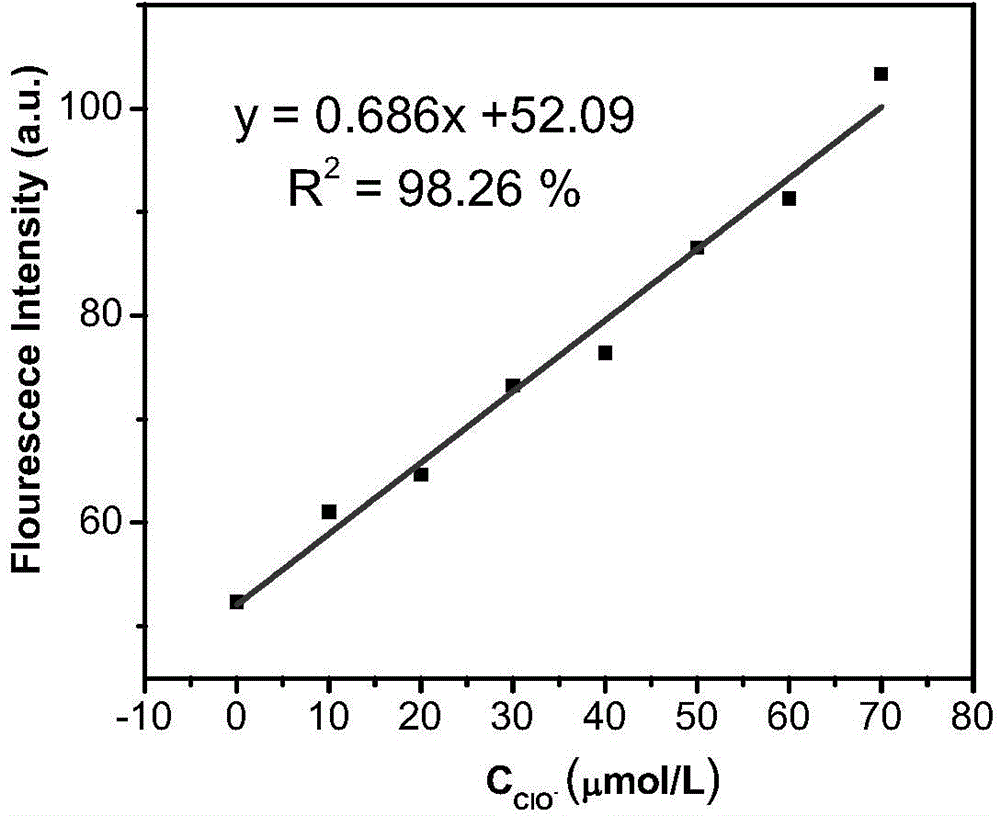
[0060] Application of fluorescence-enhanced detection of hypochlorite fluorescent probe:
[0061] Dissolve the fluorescent probe in acetonitrile to obtain a probe solution (0.02 mol / L), then add it into a PBS buffer solution (100 mM, pH=7.0), and shake well to obtain a probe test solution. Add corresponding test ions to prepare a probe colloid solution containing 5 μM probe molecules and different concentrations of test ions, and test its fluorescence spectrum changes. Figure 1-Figure 8It shows that the probe has high selectivity to hypochlorite ions, and its fluorescence spectrum changes significantly with the increase of hypochlorite ion concentration. After adding hypochlorite ions, the fluorescence intensity is enhanced by 88 times, and the probe is not affected by Effect of other common anions such as: ClO 3 - ,ClO 4 - , F - , Cl - ,Br - , SO 4 2- ,NO 3 - ,PO 4 3- ,HPO 4 2- ,CO 3 2- , HCO 3 - . In the presence of the above-mentioned interfering ions, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com