Synthesis method of 2, 2'-dihydroxy-4, 4'-dimethoxybenzophenone
A technology of dimethoxybenzophenone and a synthesis method is applied in the synthesis field of water-soluble ultraviolet absorbers, and can solve the problems of unsafe operation, high investment cost, highly toxic phosgene and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
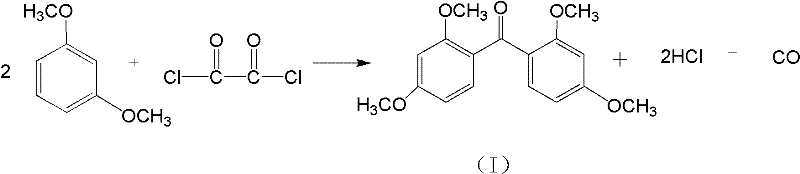
[0019] Synthesis of 2,2'-dihydroxy-4,4'-dimethoxybenzophenone, the operation steps are as follows:
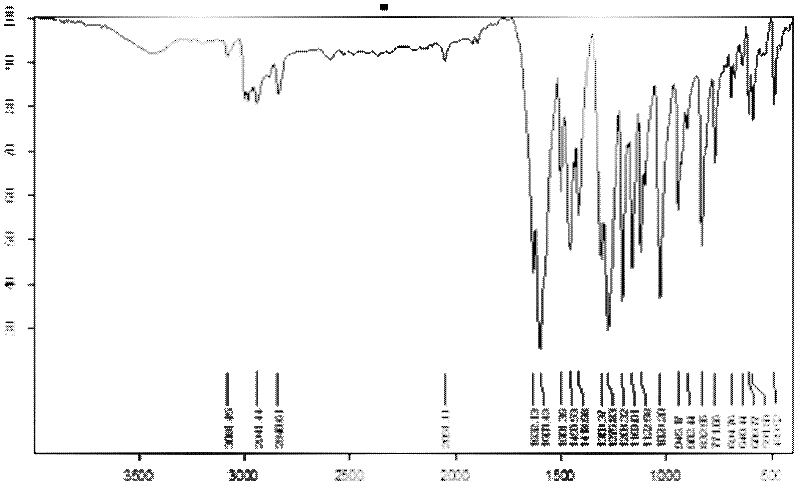
[0020] The first reaction: Take 13.8g of isophthalic ether and place it in a three-necked flask, add 0.15g of azoisobutyronitrile as a catalyst, add magnets and set up the device; add 30ml of oxalyl chloride to the flask, adjust the temperature and Stirring; Control the temperature at 70-80℃ and react for 1.5 hours; stop the reaction, add water to hydrolyze oxalyl chloride, filter and collect the intermediate product and dry, the intermediate product 2,2'4,4'-tetramethoxybenzophenone infrared The map is like figure 1 , 1 HNMR (400MHz, DMSO-d6) δ: 7.35-7.30 (m, 2H), 6.56 (s, 4H), 3.81 (s, 6H), 3.58 (s, 6H);
[0021] The second step reaction: 30g AlCl 3 And the intermediate product of the first step in the flask, then add 100ml of dichloroethane, stir and control the temperature; control the temperature at 50°C for 2-3 hours; after stopping the reaction, add water for hydrolysis, liqu...
Embodiment 2
[0023] The first step of the reaction; take 13.8g of isophthalic ether and place it in a three-necked flask, add 0.16g of azoisobutyronitrile catalyst, add magnets and set up the device; add 30ml of oxalyl chloride to the flask, adjust the temperature and stir ; Control the temperature at 70-80℃ and react for 1.5 hours; stop the reaction, add water to hydrolyze oxalyl chloride, filter and collect the intermediate product and dry; the intermediate product 2,2'4,4'-tetramethoxybenzophenone infrared spectrum Such as figure 1 ;
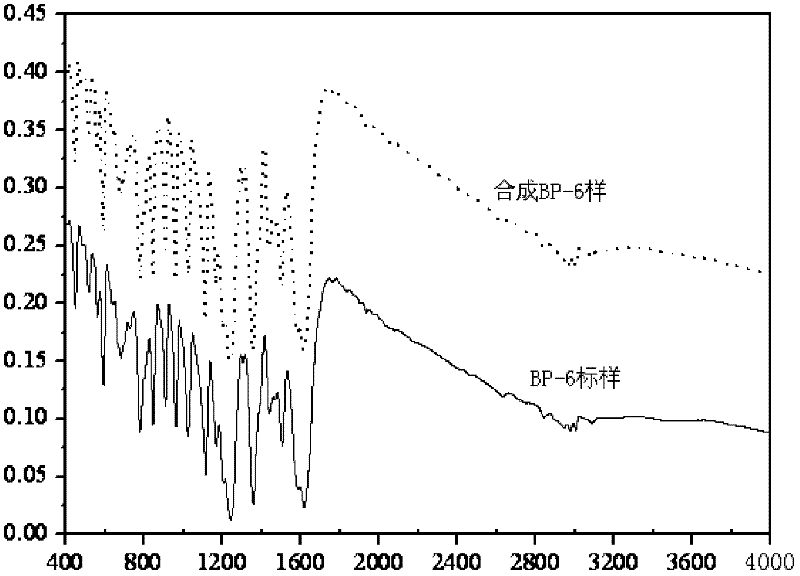
[0024] The second step of the reaction: 30g ZnCl 2 And the intermediate product of the first step in the flask, then add 100ml of dichloroethane, stir and control the temperature; control the temperature at about 50℃ for 2-3 hours; after stopping the reaction, add water for hydrolysis, liquid separation, rotary evaporation, By recrystallization, the product 2,2'-dihydroxy-4,4'-dimethoxybenzophenone was obtained, the content detected by HPLC was 99.1%, and th...
Embodiment 3
[0026] The first reaction: Take 13.8g of isophthalic ether and place it in a three-necked flask, add 0.14g of azoisobutyronitrile catalyst, add magnets and set up the device; add 30ml of oxalyl chloride to the flask, adjust the temperature and stir ; Control the temperature at 70-80℃ and react for 1.5 hours; stop the reaction, add water to hydrolyze oxalyl chloride, filter and collect the intermediate product and dry; the intermediate product 2,2'4,4'-tetramethoxybenzophenone infrared spectrum Such as figure 1 ;
[0027] The second step reaction: 30g AlCl 3 Put 100ml of chlorobenzene with the intermediate product of the first step in a flask, stir and control the temperature: control the temperature at 50°C for 2-3 hours; after stopping the reaction, add water for hydrolysis, liquid separation, rotary evaporation and recrystallization, The product 2,2'-dihydroxy-4,4'-dimethoxybenzophenone was obtained, the content detected by HPLC was 99.0%, and the yield was 67%. The infrared s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com