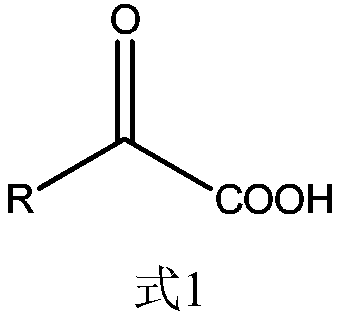
Method for synthesizing ursodeoxycholic acid and high-chiral-purity D-amino acid based on enzyme-method coupling technology
A technology of ursodeoxycholic acid and chenodeoxycholic acid, which is applied in the field of biocatalysis, can solve the problems of complicated treatment process, large environmental pollution, and high cost, achieve mild reaction conditions, simplify the reaction process, and reduce the cost of enzymes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
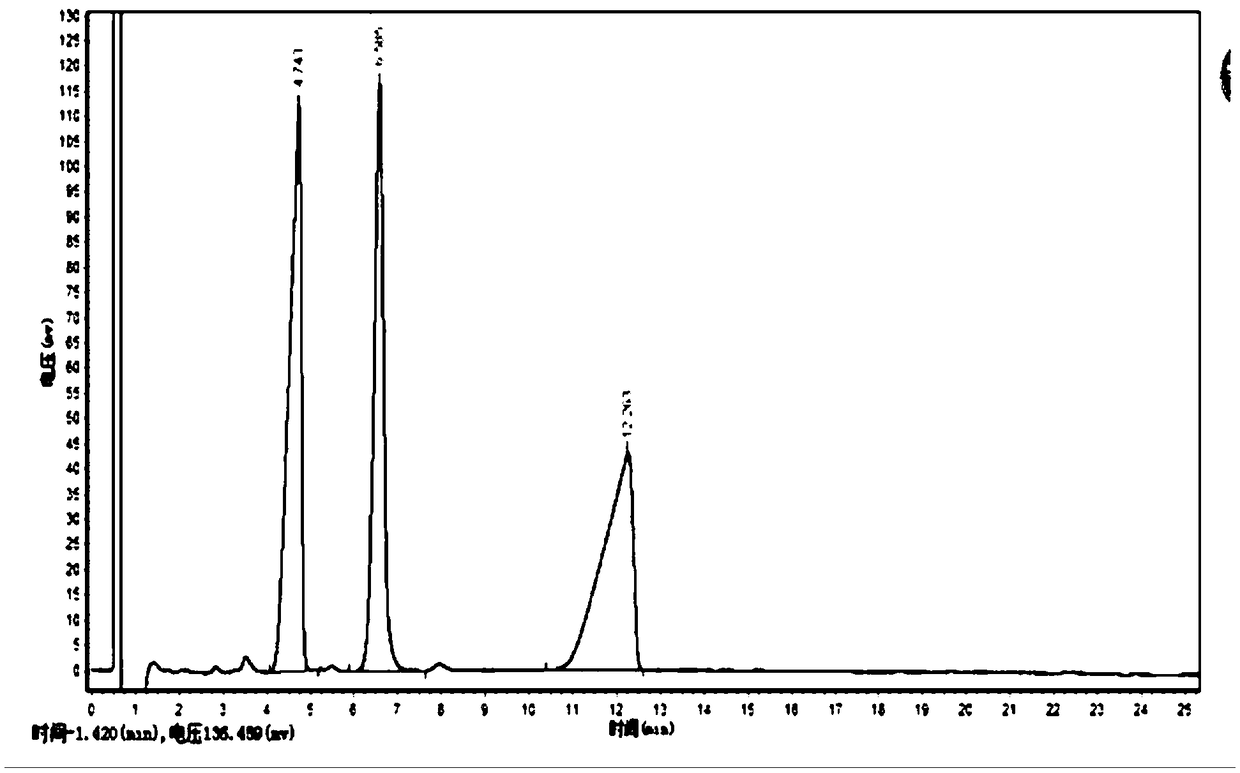
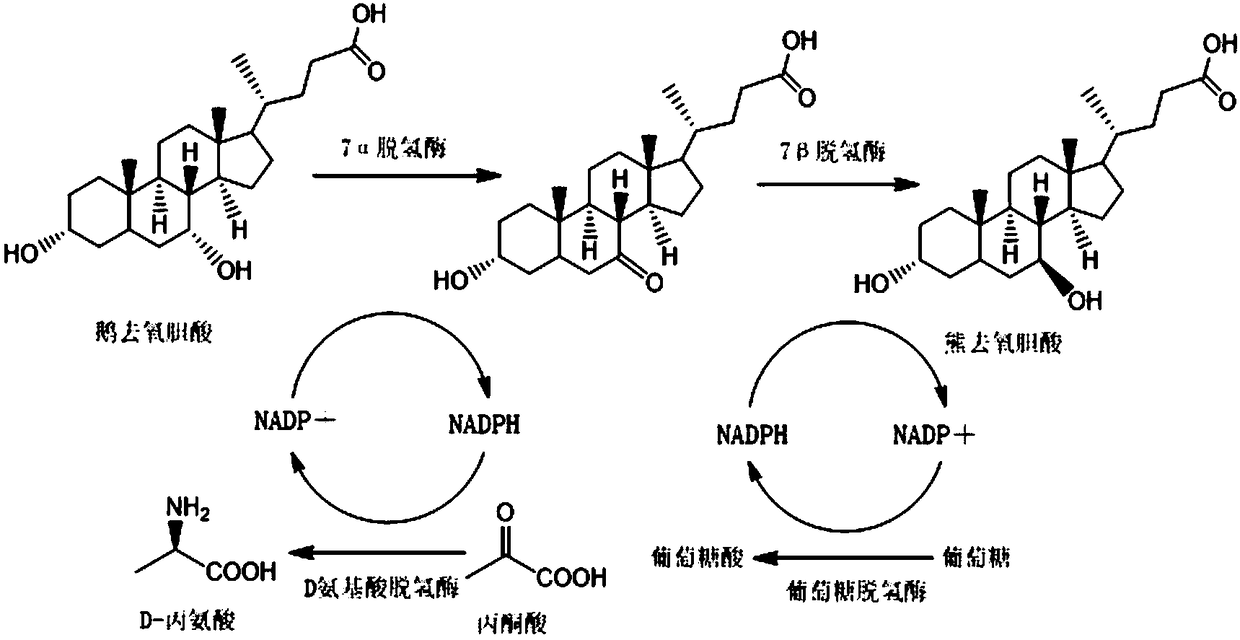
[0066] 1. Add 40.0g CDCA to the reactor, add part of deionized water to stir, adjust the pH to 8.0 with liquid caustic soda to dissolve, then add 1.2 times the molar amount of CDCA butanic acid, add deionized water to the concentration of CDCA 4%, then add 7α-HSDH liquid enzyme, DAADH liquid enzyme and NADP to react, wherein the amount of 7α-HSDH liquid enzyme added is 0.5 times the mass of CDCA*1Mu / kg, and the amount of DAADH liquid enzyme added is 0.5 times the mass of CDCA *1Mu / kg, the dosage of NADP is 2‰ of the mass of CDCA, the reaction temperature is controlled at 30°C, pH8.0, the process is monitored by HPLC until the CDCA reaction concentration is ≤0.1mg / ml, and the total reaction is 6h.
[0067] 2. The above-mentioned conversion solution is separated by a 5K ultrafiltration membrane, and the concentrated liquid enzyme is used to catalyze the next batch of CDCA. Adjust the pH of the dialysate to 3 to crystallize, and obtain a wet powder containing 7-KLCA after filtrat...
Embodiment 2
[0072] 1. Add 50.0g CDCA to the reactor, add part of deionized water to stir, adjust the pH to 8.0 with liquid caustic soda to dissolve, then add pyruvic acid with 2.5 times the molar amount of CDCA, and add deionized water to make the concentration of CDCA to be 5%, then add 7α-HSDH liquid enzyme, DAADH liquid enzyme and NADP for reaction, wherein the amount of 7α-HSDH liquid enzyme added is 0.6 times the mass of CDCA*1Mu / kg, and the amount of DAADH liquid enzyme added is 0.6 times the mass of CDCA* 1Mu / kg, the dosage of NADP is 2‰ of the mass of CDCA, the reaction temperature is controlled at 30°C, pH8.0, the process is monitored by HPLC until the CDCA reaction concentration is ≤0.1mg / ml, and the total reaction is 3h.
[0073] 2. The above-mentioned conversion solution is separated by a 5K ultrafiltration membrane, and the concentrated liquid enzyme is used to catalyze the next batch of CDCA. Adjust the pH of the dialysate to 3 to crystallize, and obtain a wet powder contain...
Embodiment 3
[0078] 1. Add 40.0g CDCA into the reactor, add part of deionized water to stir, adjust the pH to 8.0 with liquid caustic soda to dissolve, then add phenylpyruvate with 1.2 times the molar amount of CDCA, and add deionized water to set the volume to the concentration of CDCA 4%, then add 7α-HSDH liquid enzyme, DAADH liquid enzyme and NADP to react, wherein the amount of 7α-HSDH liquid enzyme added is 0.6 times the mass of CDCA*1Mu / kg, and the amount of DAADH liquid enzyme added is 0.6 times the mass of CDCA *1Mu / kg, the dosage of NADP is 2‰ of the mass of CDCA, the reaction temperature is controlled at 25°C, pH8.0, the process is monitored by HPLC until the CDCA reaction concentration is ≤0.1mg / ml, and the total reaction is 4h.
[0079] 2. The above-mentioned conversion solution is separated by a 5K ultrafiltration membrane, and the concentrated liquid enzyme is used to catalyze the next batch of CDCA. Adjust the pH of the dialysate to 6 to crystallize, and obtain a wet powder ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com