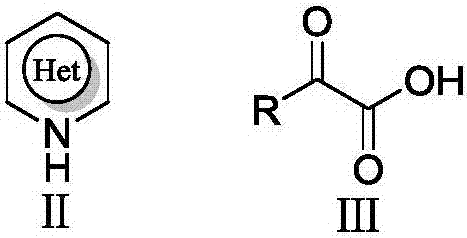
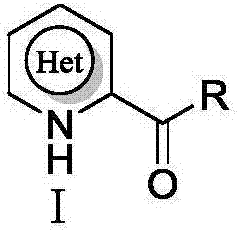
Electrochemical catalyzed synthesis method for acyl substituted electron-deficient nitrogen-containing heterocyclic compound
The technology of a nitrogen heterocyclic compound and its synthesis method, which is applied in the field of electrochemical catalytic synthesis, can solve the problems of poor chemical selectivity, incomplete reaction of raw materials, long reaction time, etc., and achieve reduced energy consumption, low equipment cost, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis under electrochemical conditions to realize the Minisci acylation reaction of α-keto acid and nitrogen-containing heterocyclic aromatic compound
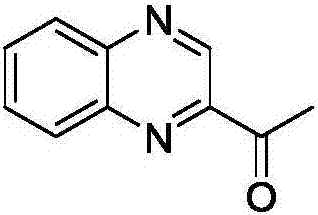
[0033] In a 50 mL single-chamber electrolytic cell, quinoxaline (1.0 mmol), pyruvic acid (3.0 mmol), HFIP (2.0 mmol) and ammonium iodide (0.15 mmol) were added to 15 mL of 0.1 M lithium perchlorate In the acetonitrile solution, with the graphite sheet electrode as the anode and the graphite sheet as the cathode, at 3mA / cm 2 Electrolyze at a constant current, stir at 70°C, stop electrolysis when the current flow reaches 2.7 F / mol, remove the solvent, dissolve with dichloromethane, wash with water three times, and separate by column chromatography to obtain acetylquinoxaline. Yield: 56%.
[0034]
[0035] yellow solid; 1 H NM R (400MHz, CDCl 3 )δ2.84(s,3H),7.82-7.90(m,2H),8.14-8.19(m,2H),9.47(s,1H);
Embodiment 2
[0036] Example 2: Synthesis under electrochemical conditions to realize the Minisci acylation reaction of α-keto acid and nitrogen-containing heterocyclic aromatic compound
[0037] In a 50mL single-chamber electrolytic cell, quinoxaline (1.0mmol), pyruvic acid (3.0mmol), and ammonium bromide (0.15mmol) were added to 15mL of acetonitrile solution dissolved in 0.1M lithium perchlorate, and graphite The sheet electrode is the anode, the graphite sheet is the cathode, at 3mA / cm 2 Electrolyze at a constant current, stir at 25°C, stop electrolysis when the current flow reaches 2.7F / mol, remove the solvent, dissolve with dichloromethane, wash with water three times, and separate by column chromatography to obtain acetylquinoxaline. Yield: 5%.
Embodiment 3
[0038] Example 3: Synthesis under electrochemical conditions to realize the Minisci acylation reaction of α-keto acid and nitrogen-containing heterocyclic aromatic compound
[0039] In a 50mL single-chamber electrolytic cell, quinoxaline (1.0mmol), pyruvic acid (3.0mmol), and ammonium bromide (0.15mmol) were added to 15mL of acetonitrile solution dissolved in 0.1M lithium perchlorate, and graphite The sheet electrode is the anode, the graphite sheet is the cathode, at 3mA / cm 2 Electrolyze at a constant current, stir at 40°C, stop electrolysis when the current flow reaches 2.7 F / mol, remove the solvent, dissolve with dichloromethane, wash with water three times, and separate by column chromatography to obtain acetylquinoxaline. Yield: 17%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com