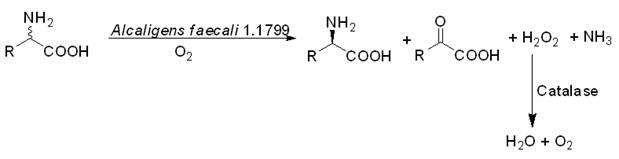
Biological catalysis method for preparing D-amino acid through deracemizing DL-amino acid
An amino acid, deracemization technology, applied in the biological field, can solve the problems of high price, poor process universality, complex separation process, etc., and achieve the effect of good versatility, easy separation, high chemical purity and optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Cell Culture
[0027] 1) Incline culture: put Alcaligens faecali 1. The 1799 strain was inoculated on sterilized solid medium and cultured statically at 30°C for 48h. Solid medium (g / L): peptone 10, beef extract 3, sodium chloride 5, agar 15, pH 7.4~7.6.
[0028] 2) Primary culture: Inoculate the cells cultured on the slant into a test tube containing 5ml of sterilized medium, and culture on a shaker at 30°C for 24 hours. Medium (g / L): peptone 10, beef extract 3, sodium chloride 5, pH 7.0.
[0029] 3) Expansion culture: Inoculate the primary cultured cells in 50ml sterile expansion medium containing inducer at 10% inoculum, and culture on a shaker at 30°C for 24h. Medium (g / L): peptone 10, beef extract 3, sodium chloride 5, L-alanine 10, pH 7.0. The culture solution was centrifuged for 15min (8000rpm) to collect the wet cells.
[0030] In step 1) above, replace with Alcaligens faecali 1.767, 1.924, 1.7686, 1.2006, 1.1837, according to the same steps...
Embodiment 2
[0031] Example 2: Cell Permeabilization Treatment
[0032] Add 50ml of 30% acetone aqueous solution to 50g of wet cells, treat at room temperature for 10min, centrifuge for 15min (8000rpm), wash twice with normal saline, and obtain 48g of permeable cells.
Embodiment 3
[0033] Embodiment 3: D-alanine preparation
[0034] The permeable cells obtained in Examples 1-2 were used as biocatalysts. Take 50g of permeabilized wet cells and suspend them in 1L of distilled water, add 17.8g (0.2mol) DL-alanine, react at 30°C for 24h, monitor the reaction with chiral HPLC until the complete conversion of L-alanine. After the reaction is completed, centrifuge for 15min (8000rpm) to remove the cells. The centrifugate was acidified with concentrated hydrochloric acid to pH 2~3, concentrated to dryness under reduced pressure, added 100ml of absolute ethanol to the residue, filtered to remove insoluble matter, added 7.0ml (0.1mol) propylene oxide to the filtrate, stirred at room temperature for 30min, filtered , the solid was washed with absolute ethanol and dried to obtain 8.0 g of D-alanine with a yield of 45% and ee of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com