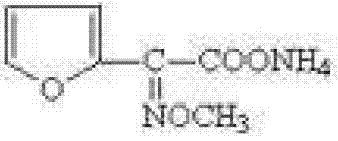
Preparation method of 2-methoxyiminofurylacetic acid amonium salt
A technology of furan ammonium salt and acetylfuran, applied in directions such as organic chemistry, can solve problems such as low conversion rate of furan ketone acid, and achieve the effects of low cost, reduced side reactions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
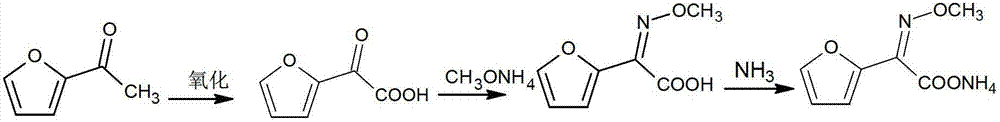
[0024] Mix and stir 200ml of water and 30g of acetylfuran, add 0.5g of copper sulfate, then add 85ml of hydrochloric acid, control the temperature at 75~80°C, add dropwise sodium nitrite aqueous solution (80g of sodium nitrite mixed with 330ml of water), after the dropwise addition is completed , adding hydrochloric acid to adjust the pH value to 3, to obtain furanone acid solution, liquid phase detection, the conversion rate is 95.17%; the solution is mixed with 70ml methoxyammonium salt, hydrochloric acid is adjusted to pH value 2, through decolorization, extraction, crystallization, refining 33 g of furan ammonium salt product was obtained.
Embodiment 2
[0026] Mix and stir 200ml of water and 30g of acetylfuran, add 0.5g of ferric sulfate, then add 85ml of hydrochloric acid, control the temperature at 40~45°C, add dropwise sodium nitrite aqueous solution (80g of sodium nitrite mixed with 330ml of water), after the dropwise addition is completed , adding hydrochloric acid to adjust the pH value to 2, to obtain furanone acid solution, liquid phase detection, the conversion rate is 94.35%; the solution is mixed with 70ml methoxyammonium salt, hydrochloric acid is adjusted to pH value 3, through decolorization, extraction, crystallization, refining 32.6 g of furan ammonium salt product was obtained.
Embodiment 3
[0028] Mix 200ml of water and 30g of acetylfuran, stir, add 0.5g of zinc sulfate, then add 85ml of hydrochloric acid, control the temperature at 60~65°C, add dropwise sodium nitrite aqueous solution (80g of sodium nitrite mixed with 330ml of water), dropwise addition is complete Finally, add hydrochloric acid to adjust the pH value to 2 to obtain furanone acid solution, liquid phase detection, conversion rate 95.35%; Refined to obtain 33.6 g of furan ammonium salt product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com