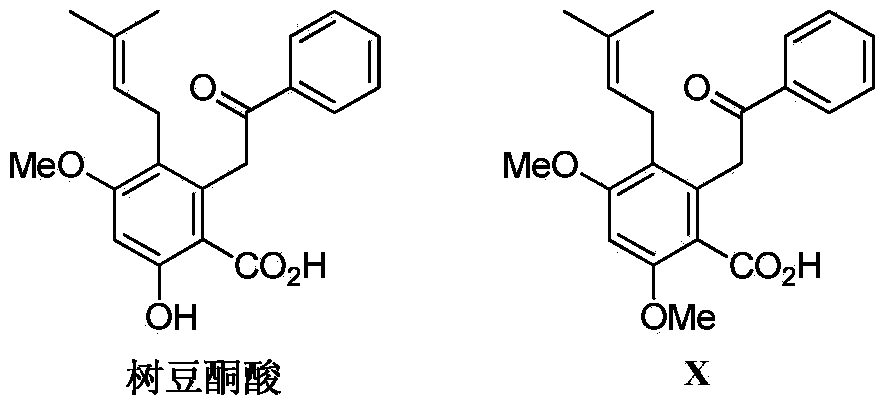
Synthesis method of diethylstilbestrol compound methyl pigeon pea ketonic acid A
A technology of methyl stearic acid and a synthesis method, applied in the field of drug synthesis, can solve the problems of difficult compound extraction and utilization, difficulty in large-scale planting, and inability to realize industrial application and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
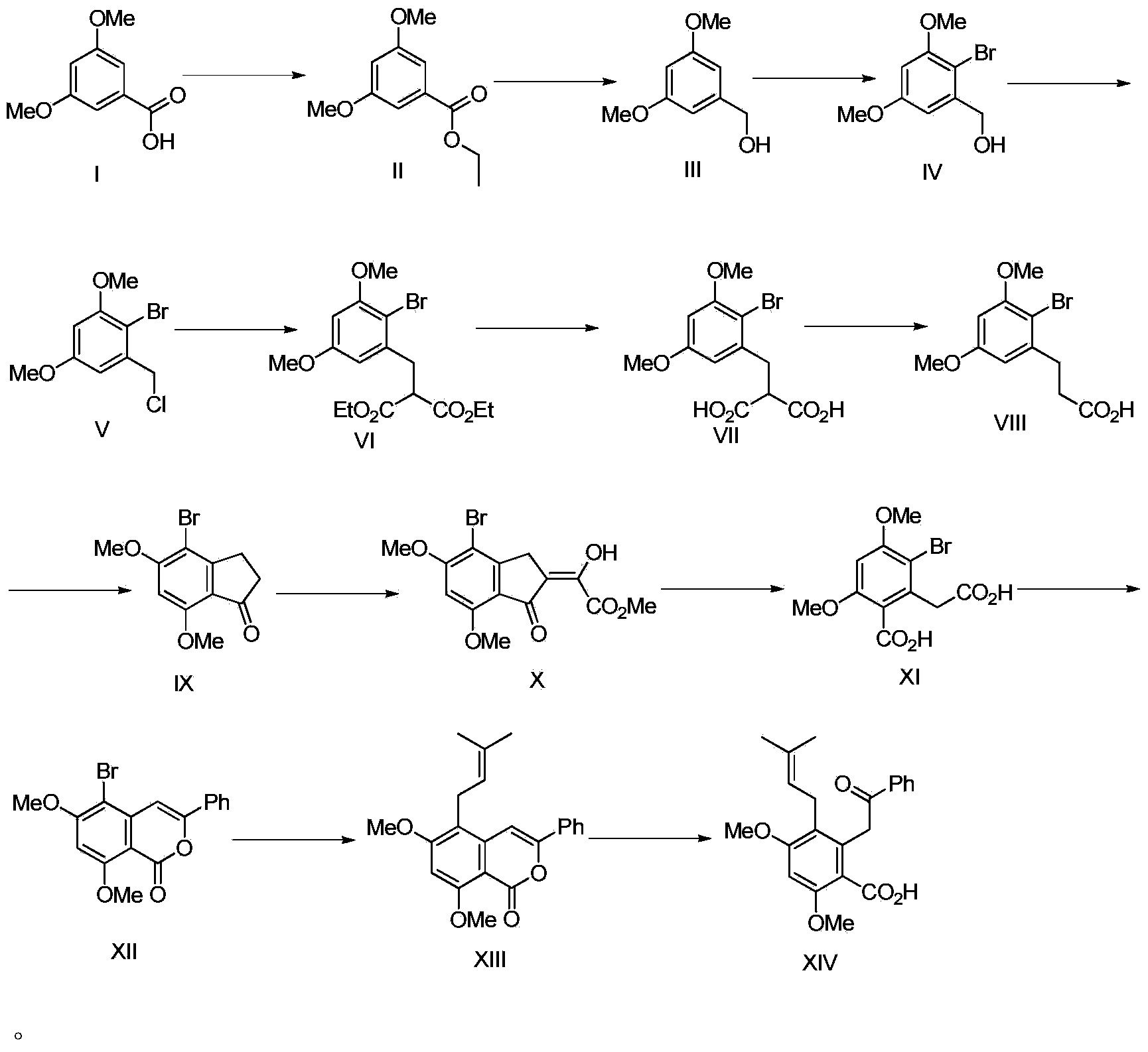
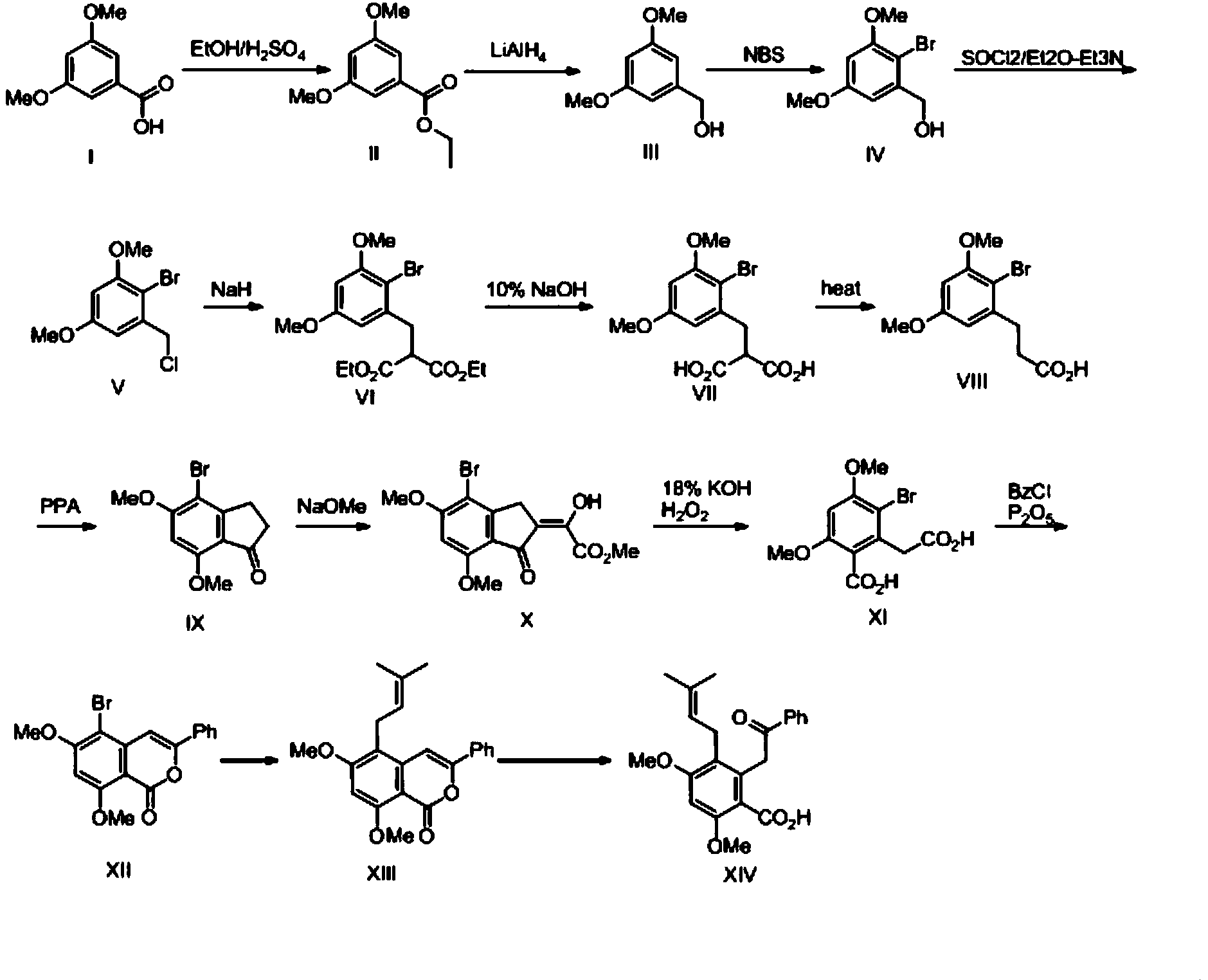
[0048] This embodiment provides a method for synthesizing methyl garbusic acid A, a columbarium compound, for synthesizing methyl jacaronic acid A, including the following processes and steps:
[0049]
[0050] 1) Preparation of compound II:
[0051] The compound of formula I (50g) was dissolved in absolute ethanol (250mL), concentrated sulfuric acid (75mL) was added dropwise to the mixed solution, and the reaction solution was refluxed for 24 hours. After the reaction was over, the solvent was evaporated under reduced pressure, extracted 3 times with ethyl acetate, the organic phases were combined, and the organic phase was washed with 10% sodium bicarbonate solution and water for several times, then dried with anhydrous magnesium sulfate, concentrated to obtain 55.3g Colorless liquid compound with a yield of 96%. After nuclear magnetic detection, the compound was confirmed to be a compound of formula (II) after analysis. The nuclear magnetic data is as follows: 1H NMR (4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com