Atomizing agent with arformoterol and glycopyrronium bromide serving as active components and preparation method of fogging agent
A technology of glycopyrronium bromide and active ingredients, which is applied in the field of medicine and can solve the problems of poor stability and easy degradation of arformoterol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Preparation of arformoterol tartrate single atomizing agent and glycopyrrolate single atomizing agent
[0059] According to Table 1, a single atomizing agent for arformoterol tartrate and a single atomizing agent for glycopyrrolate were prepared.
[0060] Table 1
[0061]
[0062] Preparation:
[0063] Weigh the prescription amount of sodium chloride and dissolve it in an appropriate volume of water (about 700ml), stir to dissolve, add the prescription amount of arformoterol tartrate or glycopyrrolate, stir until dissolved, then add water to the full amount, add citric acid-citric acid Adjust the pH with sodium citrate buffer to obtain.
Embodiment 2
[0064] Example 2 Preparation of arformoterol tartrate and glycopyrrolate compound atomizing agent
[0065] Prepare arformoterol tartrate and glycopyrrolate compound atomizer according to Table 2.
[0066] Table 2
[0067]
[0068] Preparation:
[0069] Weigh the prescription amount of sodium chloride and dissolve it in an appropriate volume of water (about 700ml), stir to dissolve, add the prescription amount of arformoterol tartrate and glycopyrrolate, stir until dissolved, then add water to the full amount, add citric acid-citric acid Adjust the pH with sodium citrate buffer to obtain.
Embodiment 3
[0070] Example 3 Investigation of the dosage of arformoterol tartrate and glycopyrrolate in the compound atomizer
[0071] According to the preparation method of Example 2, the arformoterol tartrate and glycopyrrolate compound atomizer in Table 3 below was prepared.
[0072] table 3
[0073]
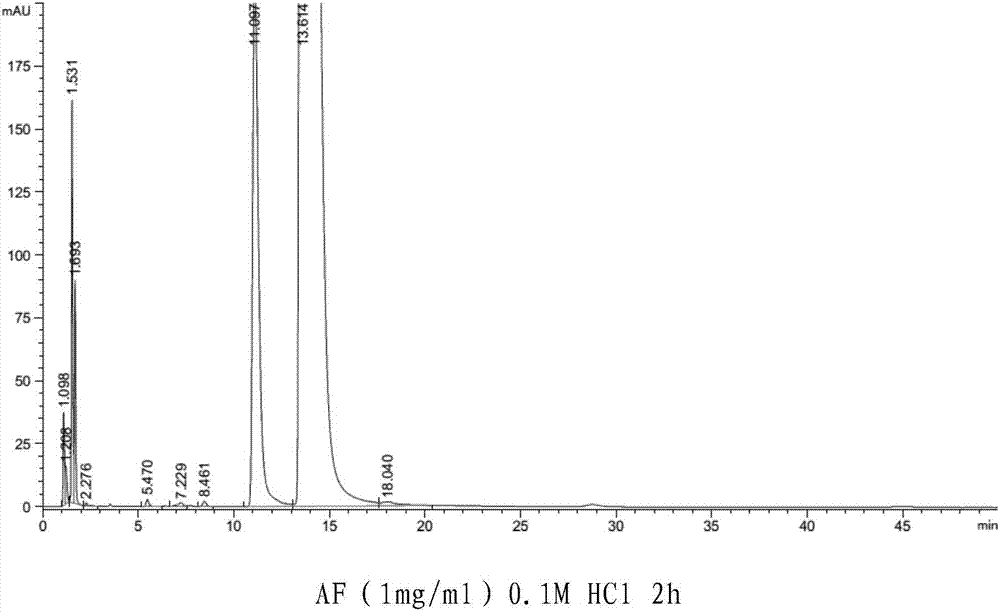
[0074] Take five compound atomizers of arformoterol tartrate and glycopyrrolate prepared according to Table 3 and place them at 40℃±2℃ / 75%RH±5%RH for 1 month, and then take samples to determine the content of the main drug T 0 The content is taken as 100%). The results of the accelerated test at 40°C±2°C / 75%RH±5%RH for one month are shown in Table 4 below.
[0075] Table 4
[0076]
[0077] The results show that, in the compound atomizing agent of arformoterol tartrate and glycopyrrolate of the present invention, the preferred dosage of arformoterol tartrate is 11 μg / mL, and the preferred dosage of glycopyrrolate is 18 μg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com