Method for preparation of glycopyrronium bromide
A technology of glycopyrronium bromide and compound, applied in the field of preparation of glycopyrronium bromide, can solve the problems of inability to guarantee safe production of glycopyrronium bromide, extremely high equipment and environmental requirements, unsuitable for industrialized production, etc., and achieves easy industrialized production , low-cost, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0044] a) Add 440g of α-cyclopentylmandelic acid and 6L of toluene to a 10L dry reaction kettle, under stirring at room temperature, add 360g of 4-chloro-2,6-dimethoxytriazine, and stir for 20min to obtain formula (II ) active intermediate solution
[0045] b) In the above solution, add 200g of 1-methyl-3-pyrrolidinol in 300ml of isopropanol solution, heat to 60°C within 1h, and keep stirring for 5 hours
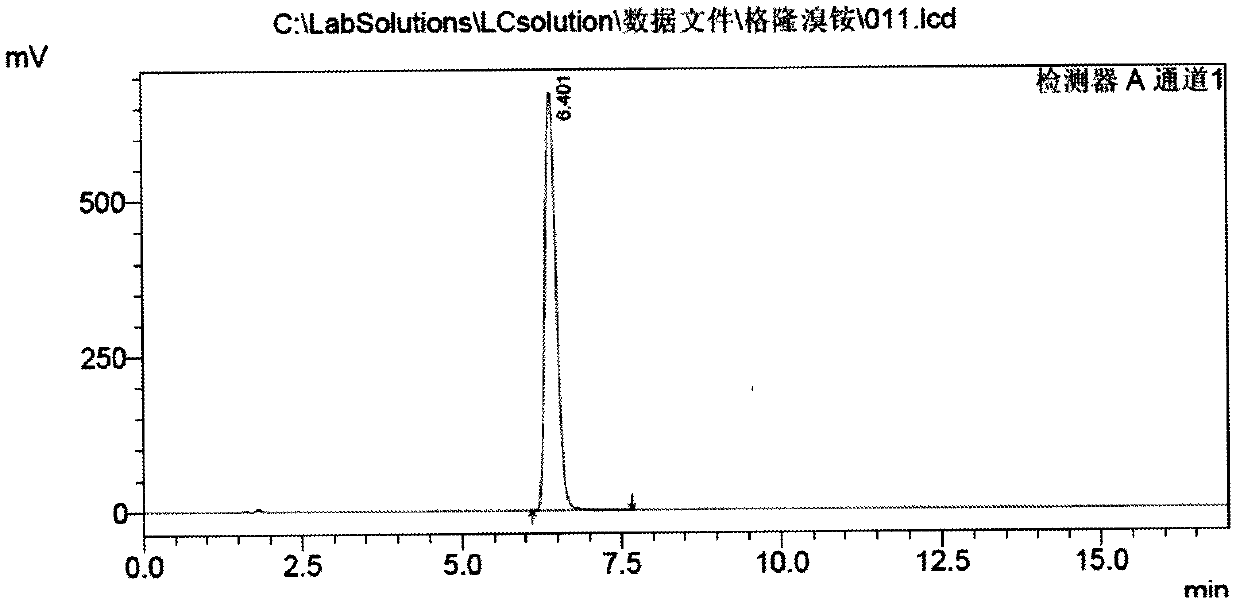
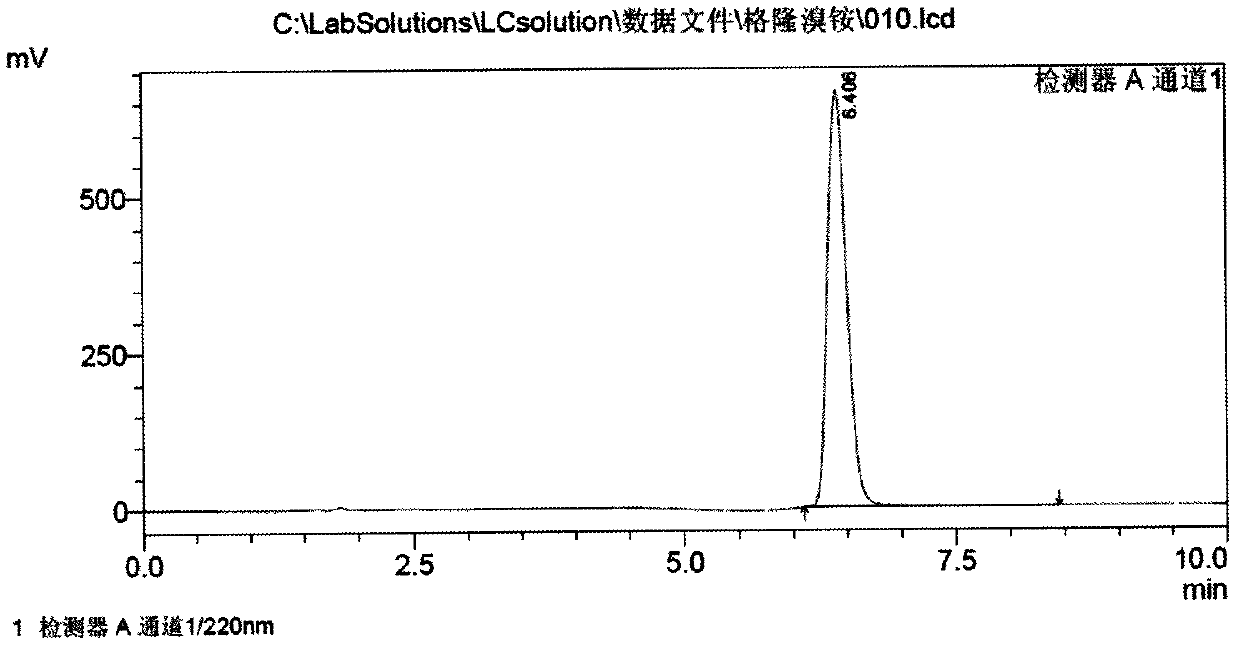
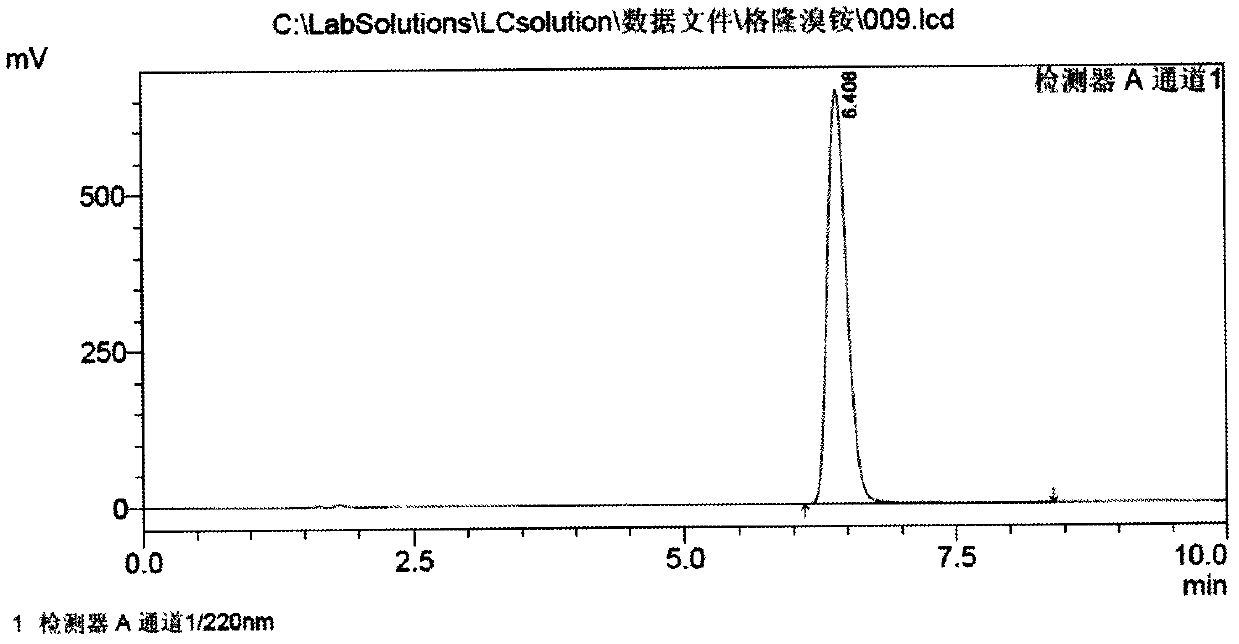
[0046] c) Cool the above solution to -20°C, add 210g of methyl bromide dropwise, keep stirring for 2h, then raise the temperature to 60°C and keep stirring for 20min, cool to room temperature, let stand for 5h, filter, wash the solid with a small amount of isopropanol, 70-80°C Vacuum drying yielded 361 g of glycopyrronium bromide. The yield is 89.5% based on α-cyclopentylmandelic acid, and the melting point is 195°C to 198°C.
[0047]
[0048] 1 H-NMR (600MHz, CDCl 3 / TMS,ppm):
[0049]δ1.07~1.09(m, 1H); δ1.28~1.31(m, 1H); δ1.37~1.43(m, 1H); δ1.45~1.53(m, 1H); δ1.57~...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com