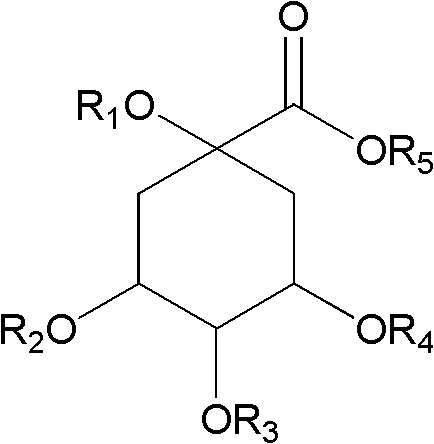
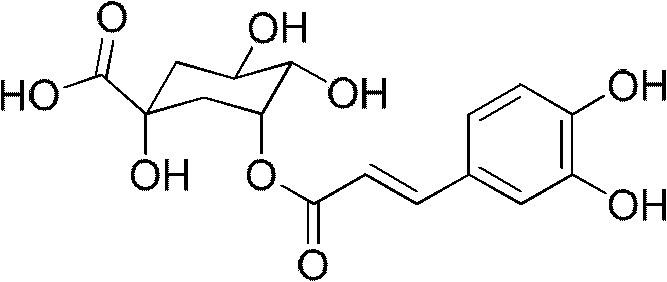
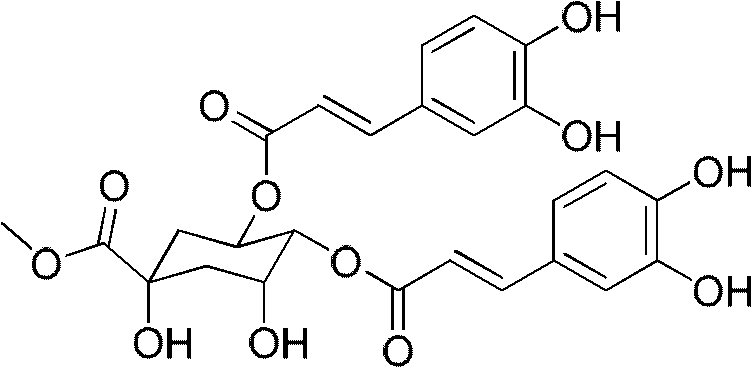
Application of caffeoylquinic acid and its derivatives in the preparation of anti-complement drugs
A technology of caffeoylquinic acid and caffeoylquinic acid methyl ester, which is applied in the field of application of caffeoylquinic acid and its derivatives in the preparation of anti-complementary drugs, and can solve the problems that have not been reported on caffeoylquinic acid, etc. problems, to achieve the effect of great clinical application value, rich sources of raw materials, and drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of caffeoylquinic acid and derivatives thereof
[0042] 25 kg of dry whole herb of S. sageii (purchased from the medicinal material market in Haozhou, Anhui, China) was pulverized, extracted 3 times under 80% ethanol hot reflux (200L×3), combined the extracts to recover ethanol, and concentrated to dryness under reduced pressure at 60°C to obtain S. sativa Grass total extract 2.9kg, take total extract 2.5kg and suspend with water (15L), sequentially extract with equal volume of petroleum ether, ethyl acetate, n-butanol for 3 times, combine ethyl acetate extract and concentrate under reduced pressure at 60°C To dryness, 450 g of ethyl acetate extract was obtained. Take 400 g of the ethyl acetate extract and pass through a silica gel column (4 kg, 100-200 mesh, 10 cm × 100 cm) chromatography, and dichloromethane: methanol (70:1, 50:1, 30:1, 20:1, 10:1 , 5:1, 3:1, 1:1) gradient elution, each gradient elution 20L, collect dichloromethane:methanol (3:1...
Embodiment 2
[0051] Example 2 classical pathway complement inhibition test
[0052] 1 Instruments and reagents
[0053] Low-temperature high-speed centrifuge (Jouan MR22i), microplate reader (Thermo Labsystems, well scanMK3), sheep red blood cells, anti-sheep red blood cell antibodies (sigma company), human serum, barbiturate-barbital sodium buffered saline (BBS 2+ , pH=7.4, containing 0.5mM Mg 2+ and 0.15mM Ca 2+ ), triple distilled water, heparin sodium, and a constant temperature water bath.
[0054] 2 test drugs
[0055] The total sage extract prepared in Example 1 and the caffeoylquinic acid compounds 1-7 isolated therefrom
[0056] 3 Experimental methods
[0057] Take human serum to VBS 2+ Buffer (barbital buffer, pH=7.4, containing 0.5mM Mg 2+ and 0.15mM Ca 2+ ) diluted 1:10, as a source of "complement" in the classical pathway. Antibodies against goat erythrocytes in VBS 2+ The buffer was diluted 1:1000 as hemolysin; sheep red blood cells were treated with VBS 2+ The buf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com