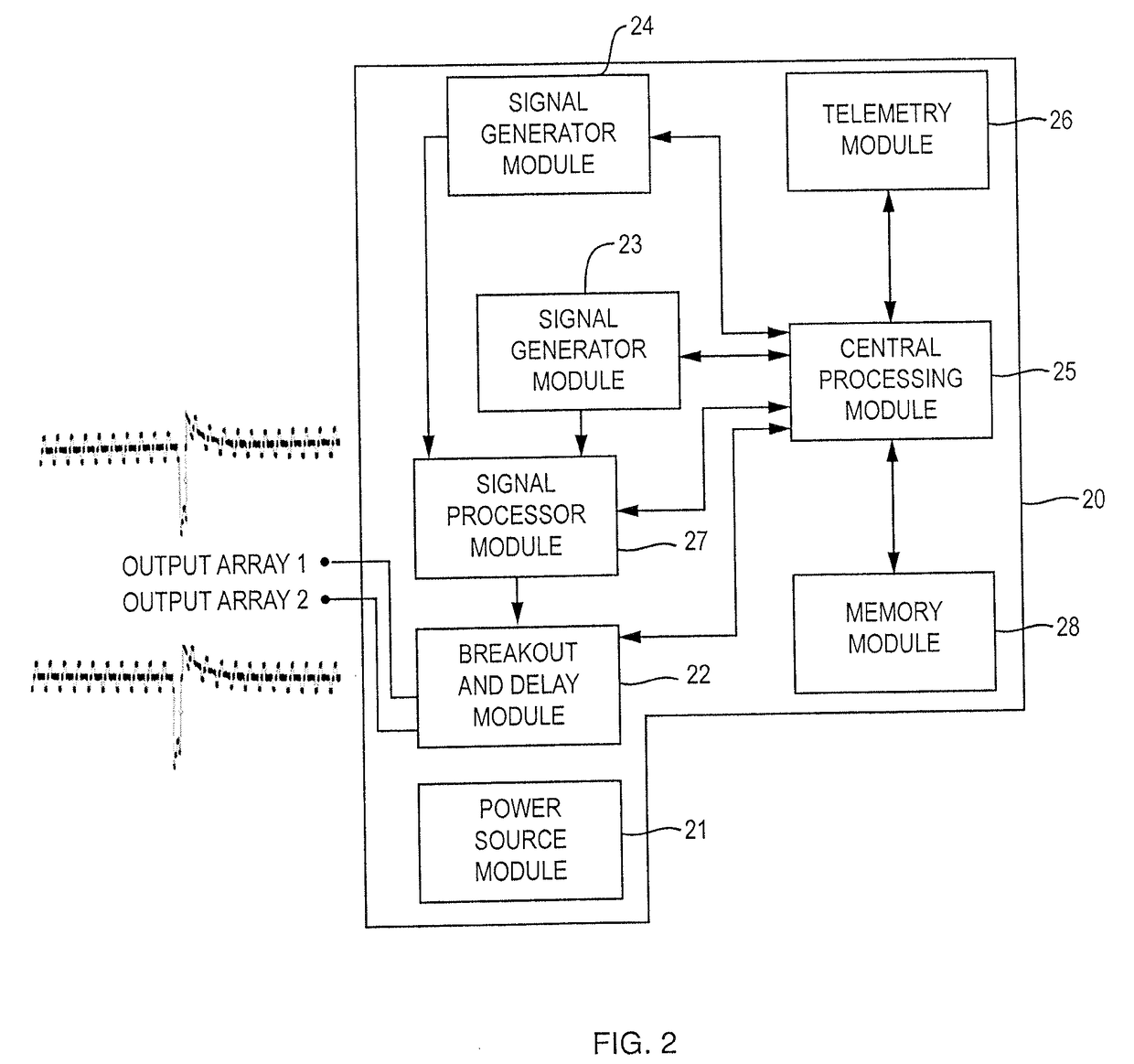
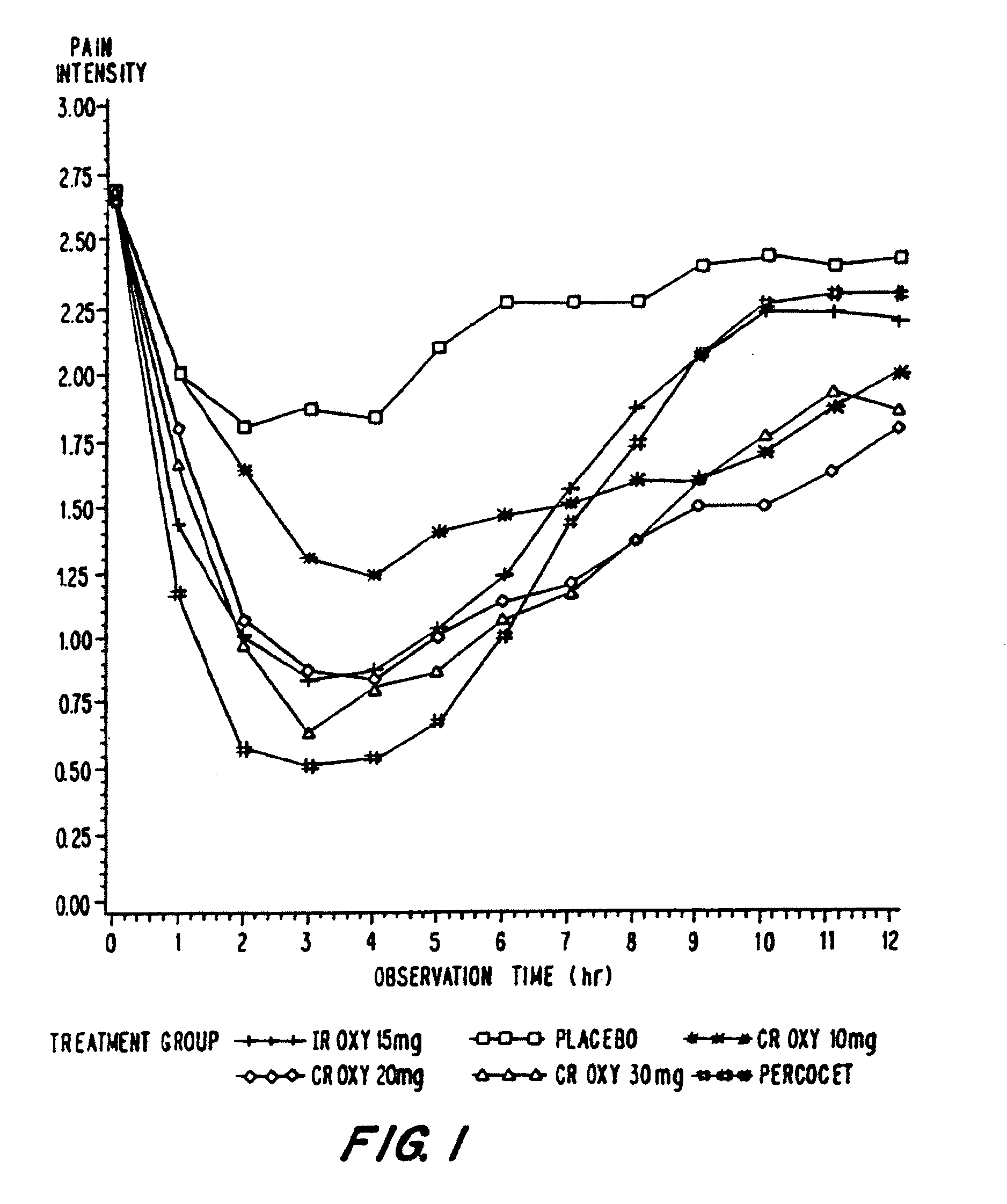
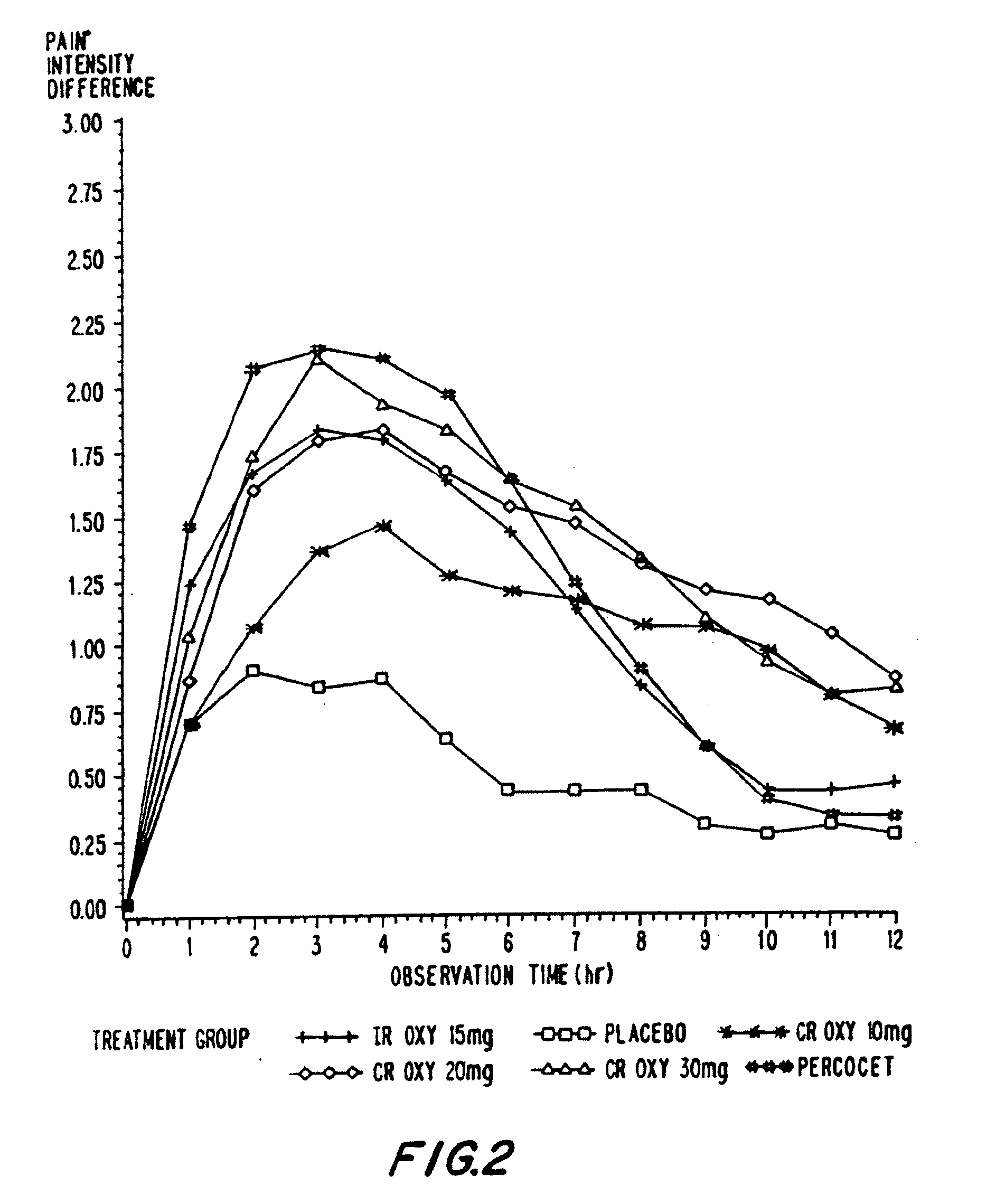
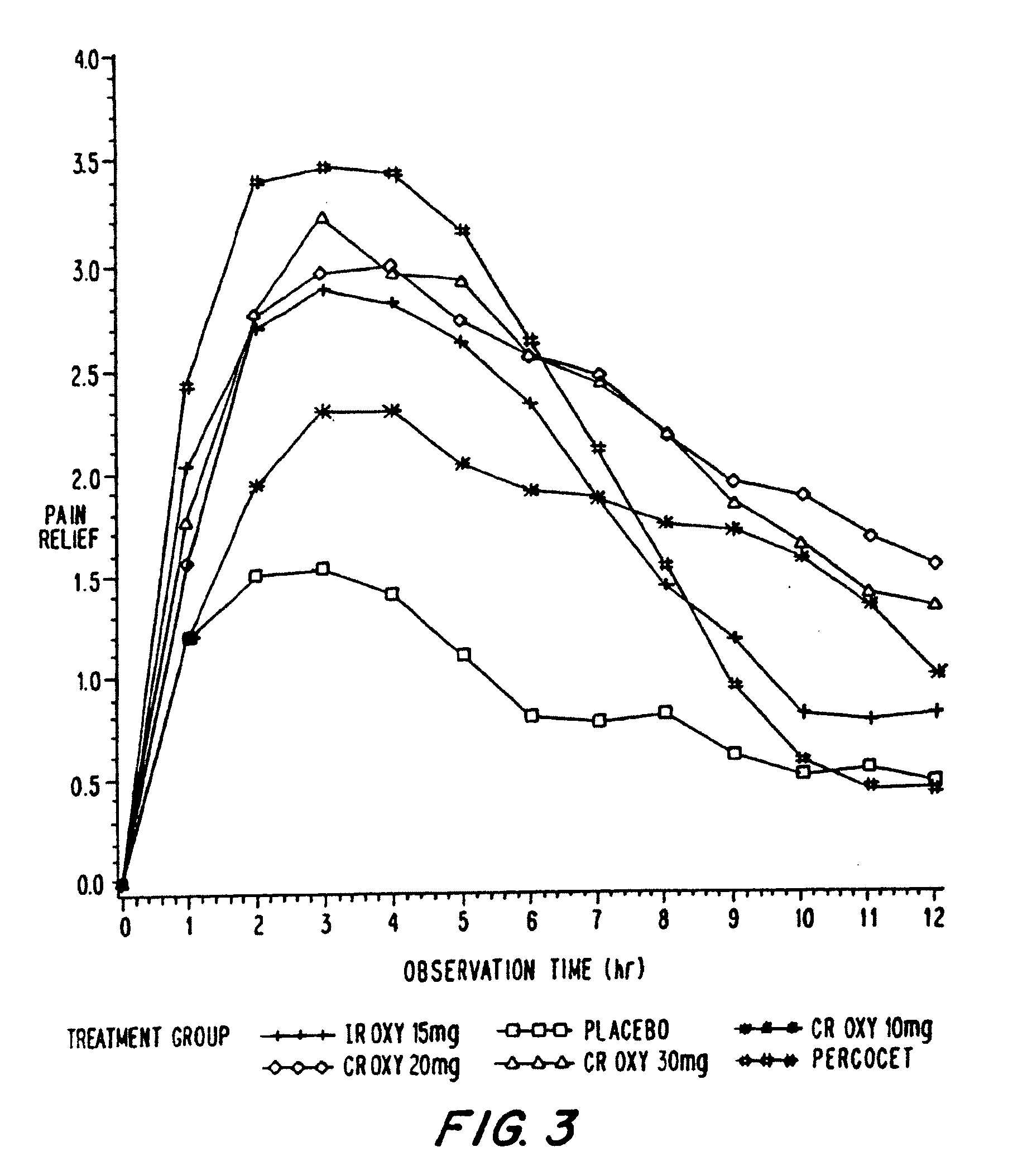
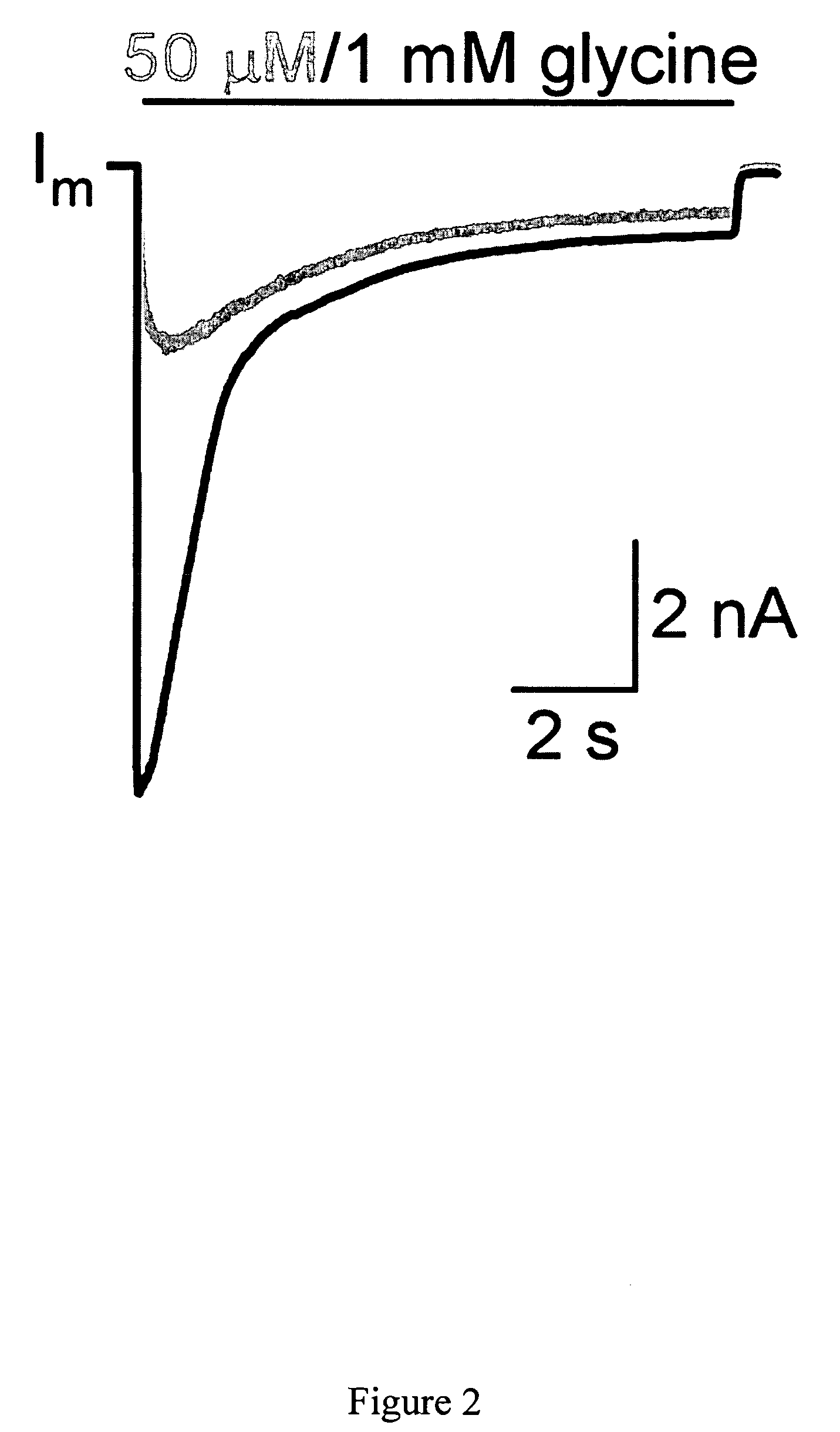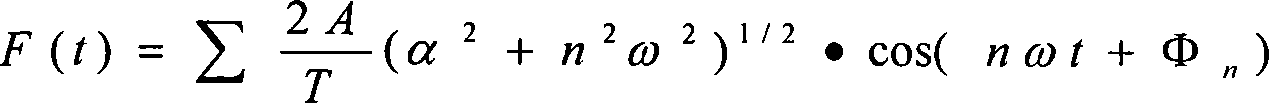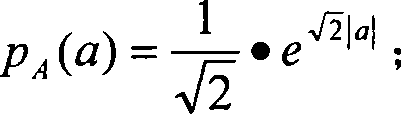Patents
Literature
45 results about "Controlled pain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multiprogrammable tissue stimulator and method
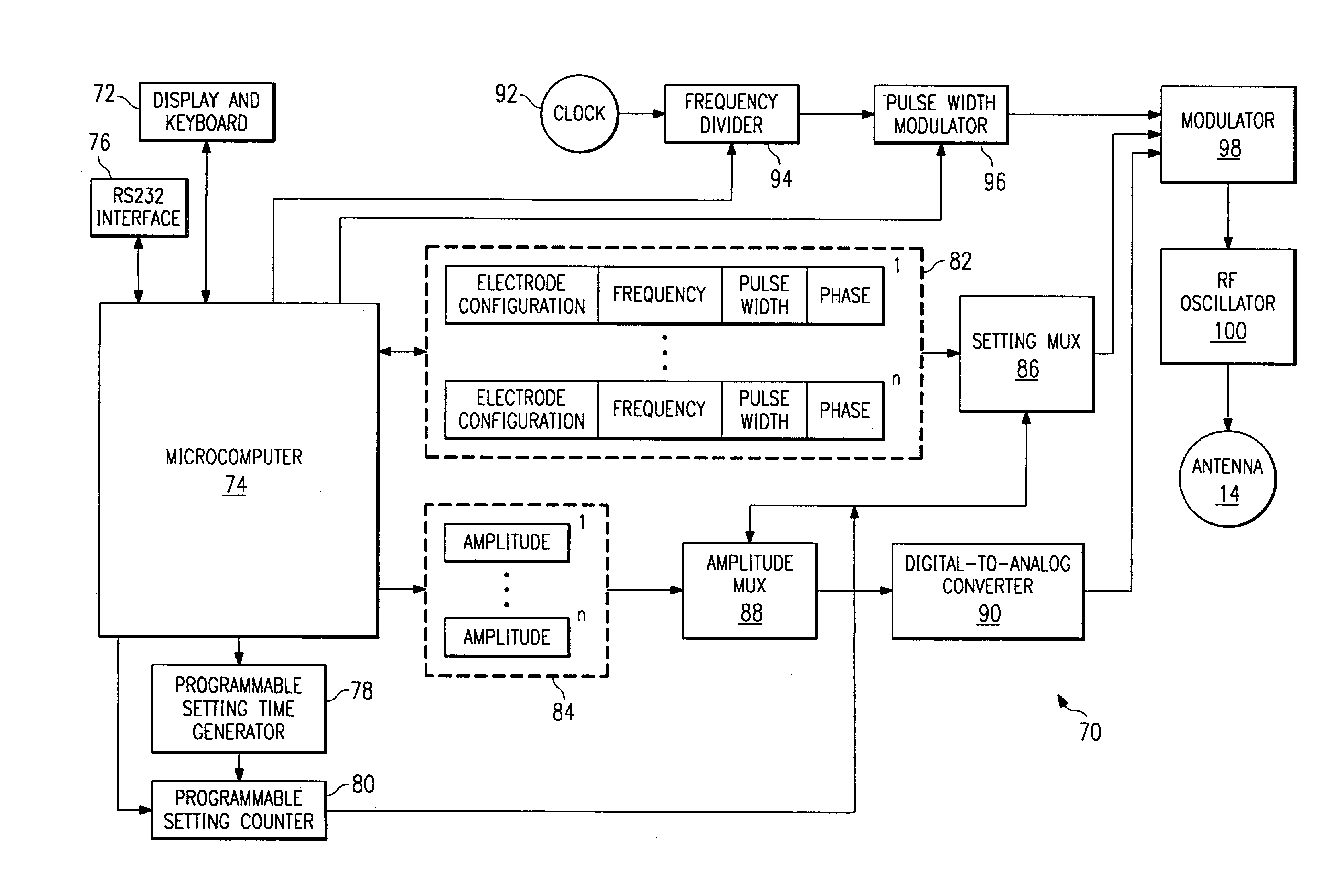
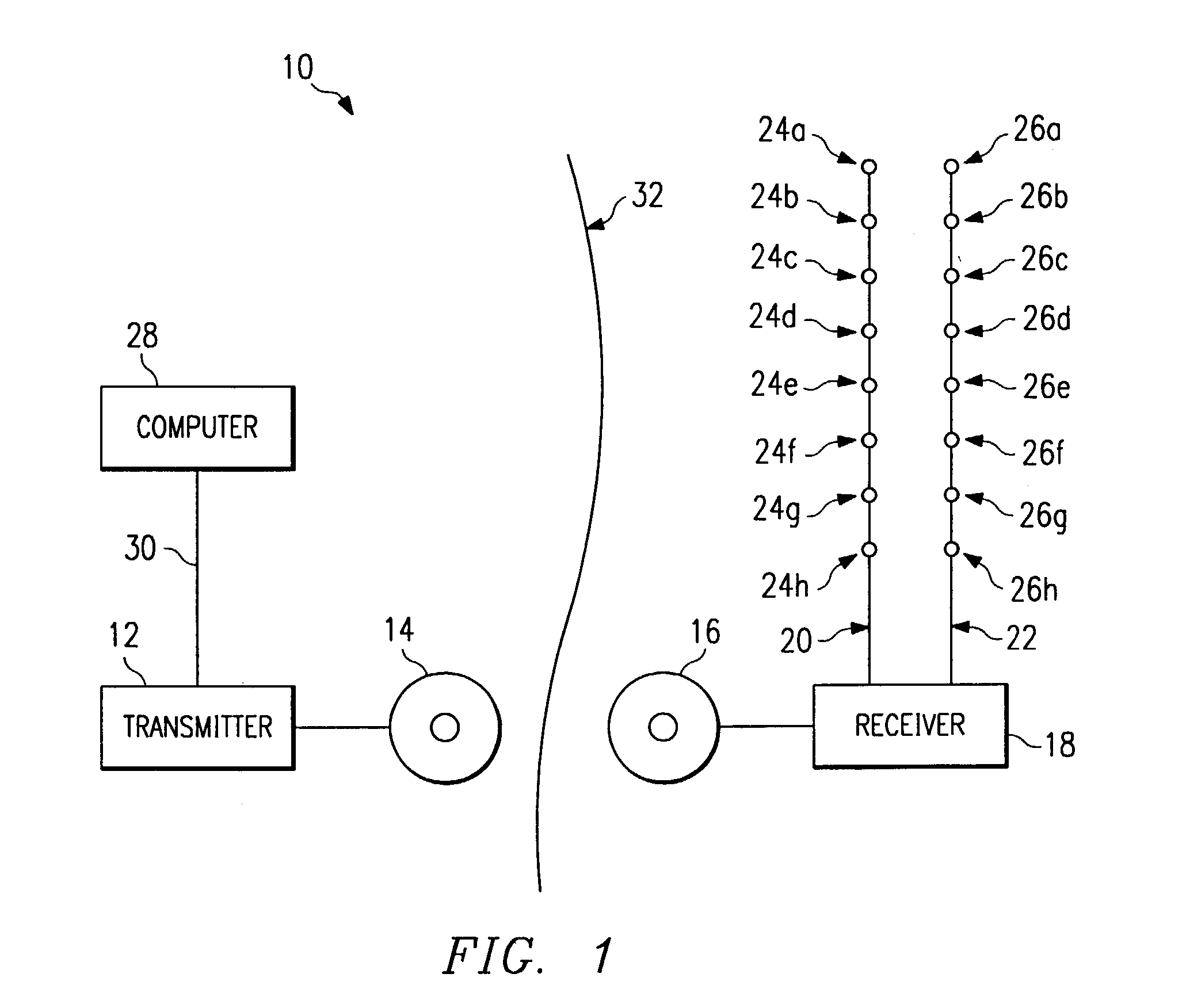
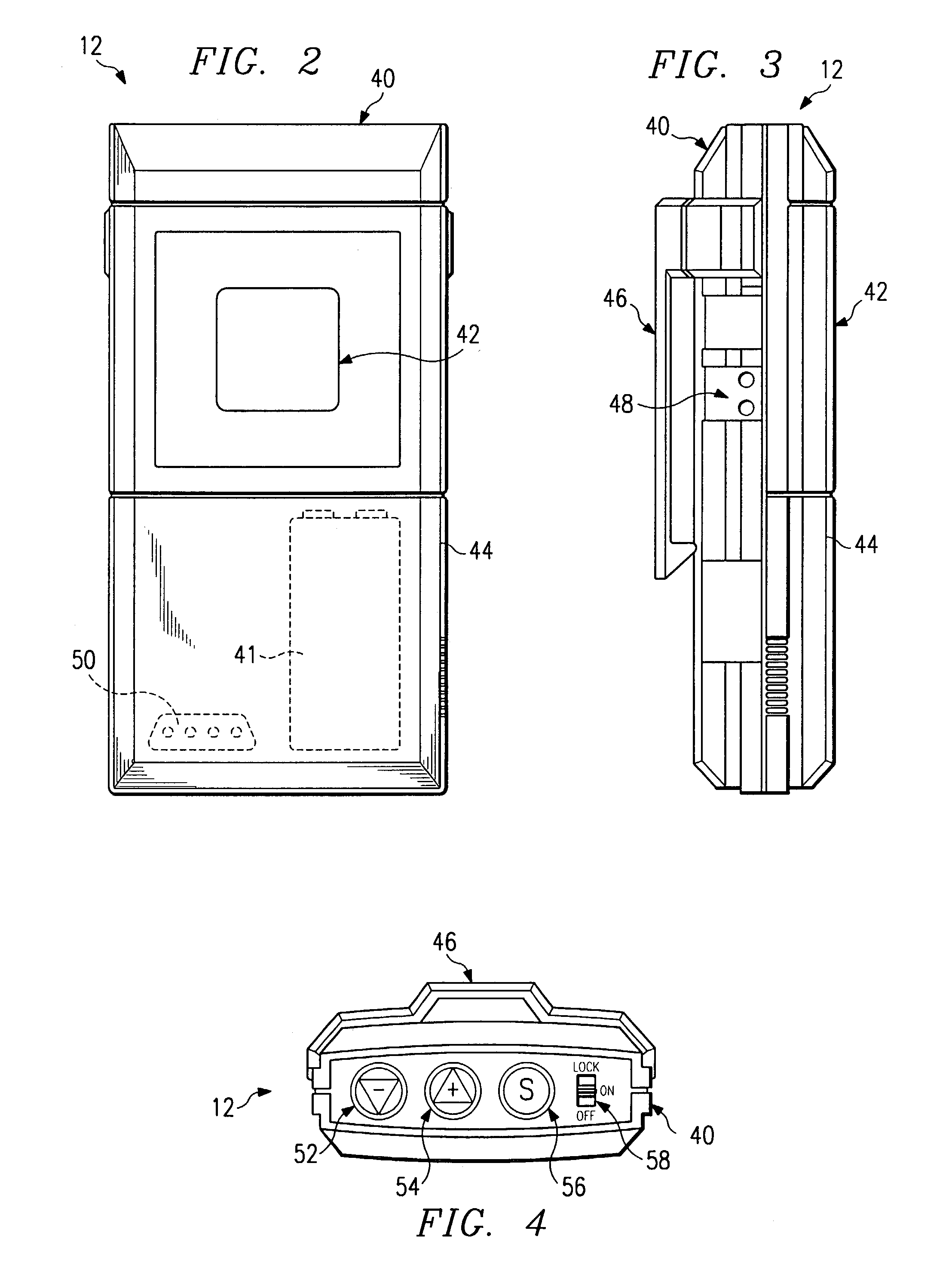
An electronic stimulation system is used to control pain over multiple regions of a patient's body. The system has one or more percutaneous leads, each having multiple electrodes, implanted within the patient's epidural space parallel to the axis of the spinal cord. The leads are connected to either a totally implanted system or a radio frequency system. The system is able to treat pain over different regions of a patient's body by “simultaneously” stimulating the patient with at least three different stimulation settings. “Simultaneous” stimulation involves sequentially stimulating the patient with the multiple stimulation settings such that the patient receives the cumulative effect of each stimulation setting, while not perceiving the transition from one stimulation setting to another.
Owner:ADVANCED NEUROMODULATION SYST INC
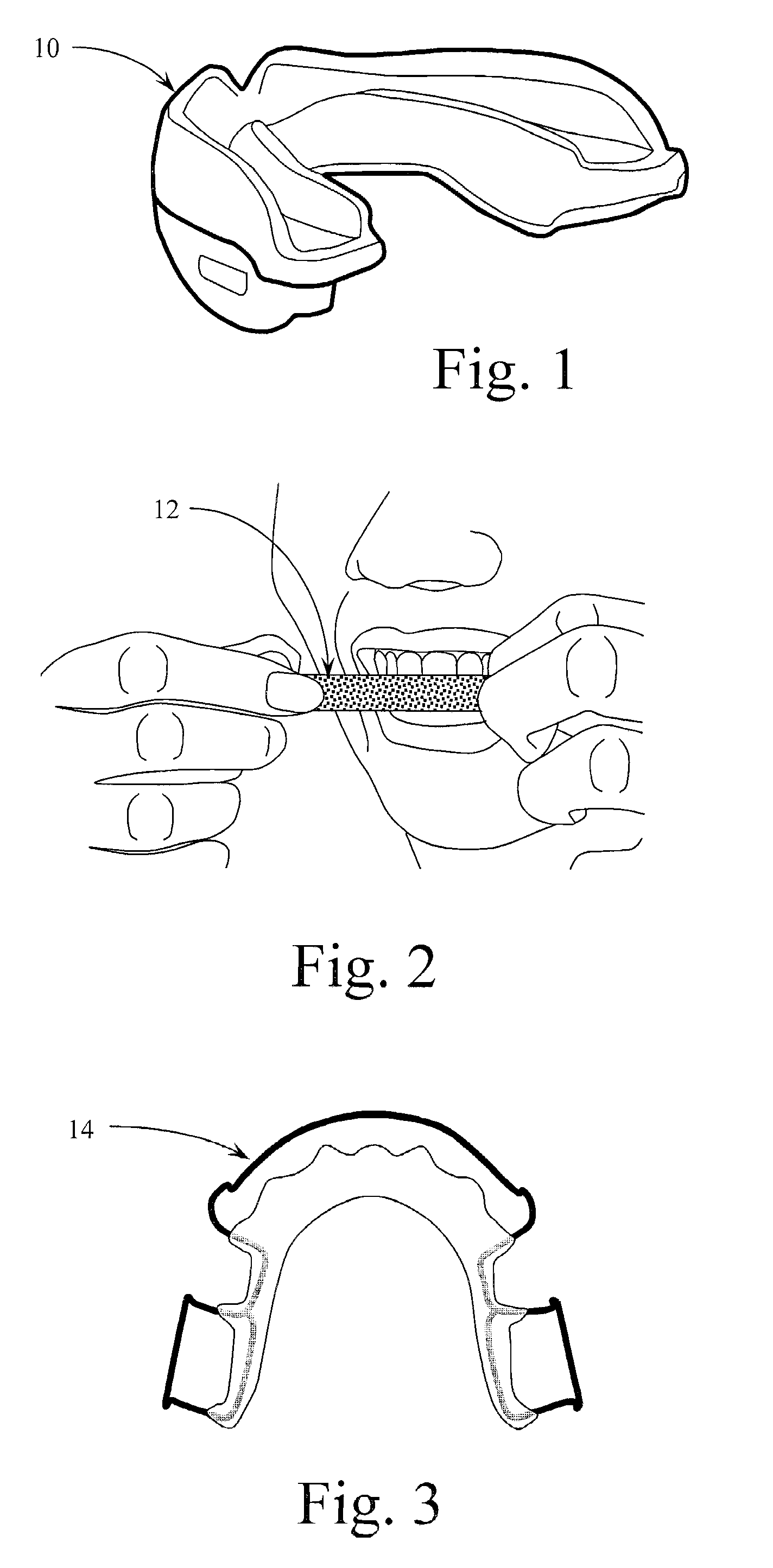
Implantable medical lead and system, and method of use thereof
InactiveUS20050021119A1Precise positioningEliminate needSpinal electrodesHead electrodesSpinal columnManaged pain
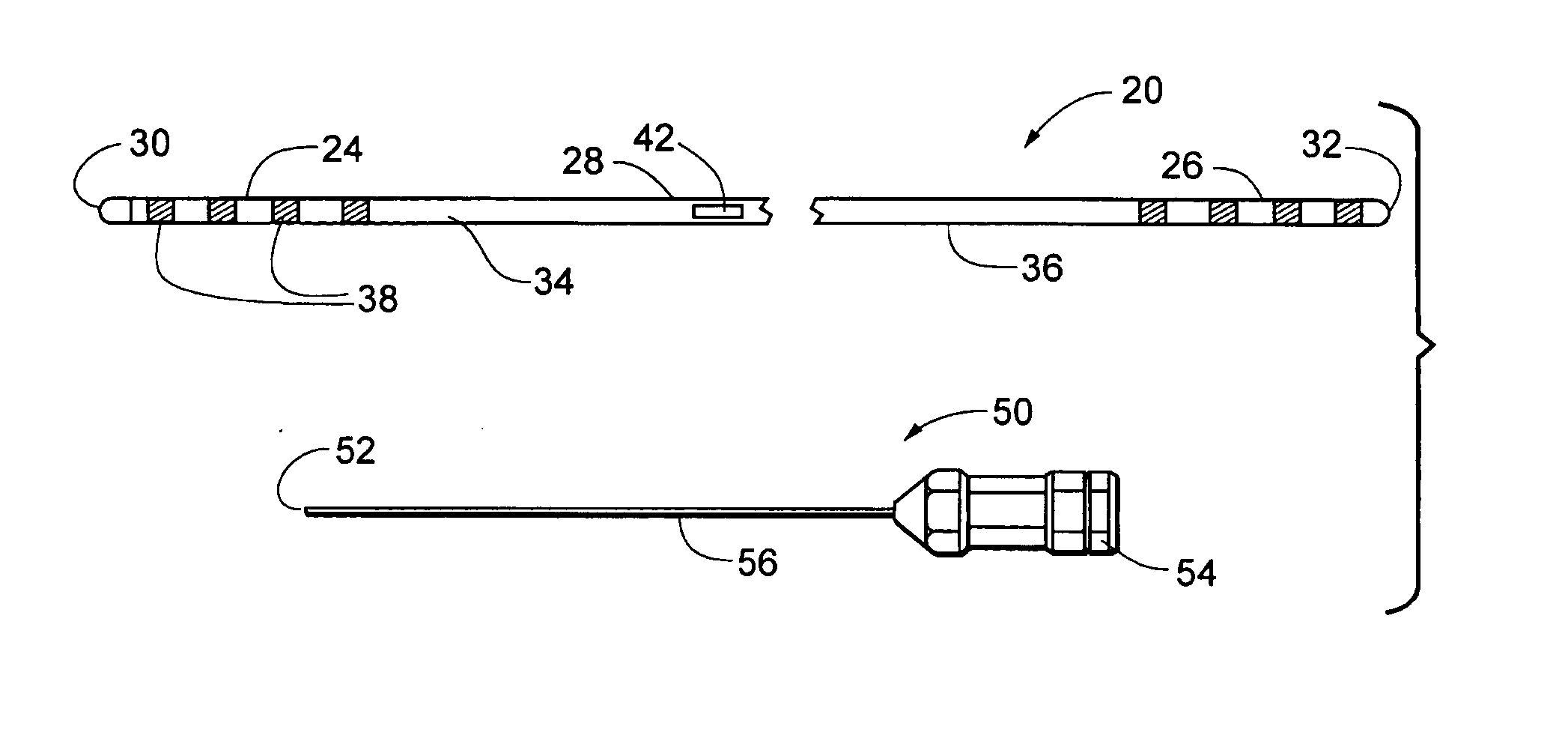
Implantable medical leads, lead assemblies, and methods for implanting the leads into the human body, for example, for use within the epidural spaces of the spinal column to manage pain. A stylet lumen extends within the lead between a side-wall stylet entrance port and a lead distal region. In use, exemplary stylets may be inserted through the lead stylet port to stiffen only the distal portion of the lead, and the distal portion of the lead inserted through an introducer needle into the human body. The stiffened distal portion of the lead may be short and easily controllable.
Owner:MEDTRONIC INC
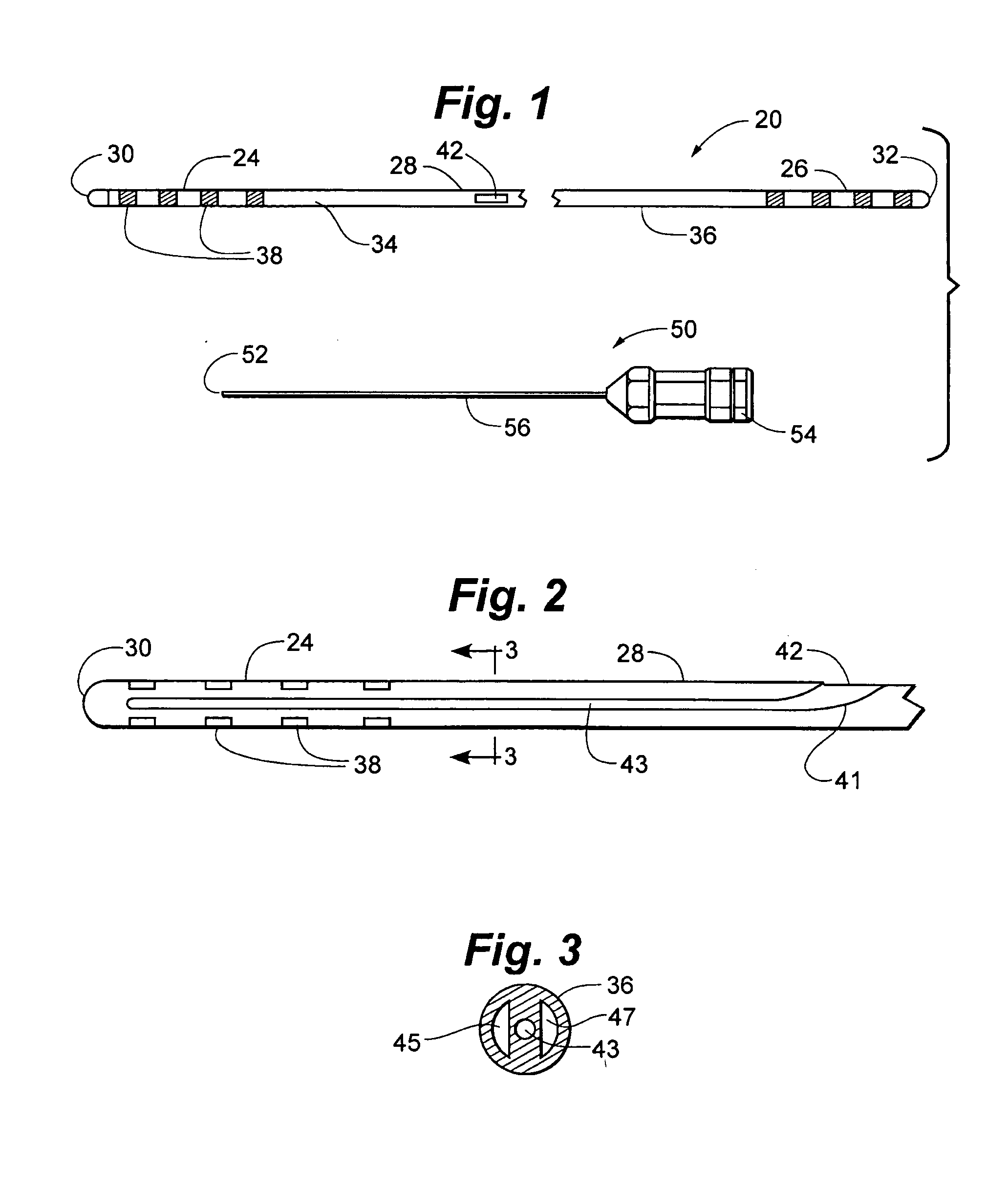
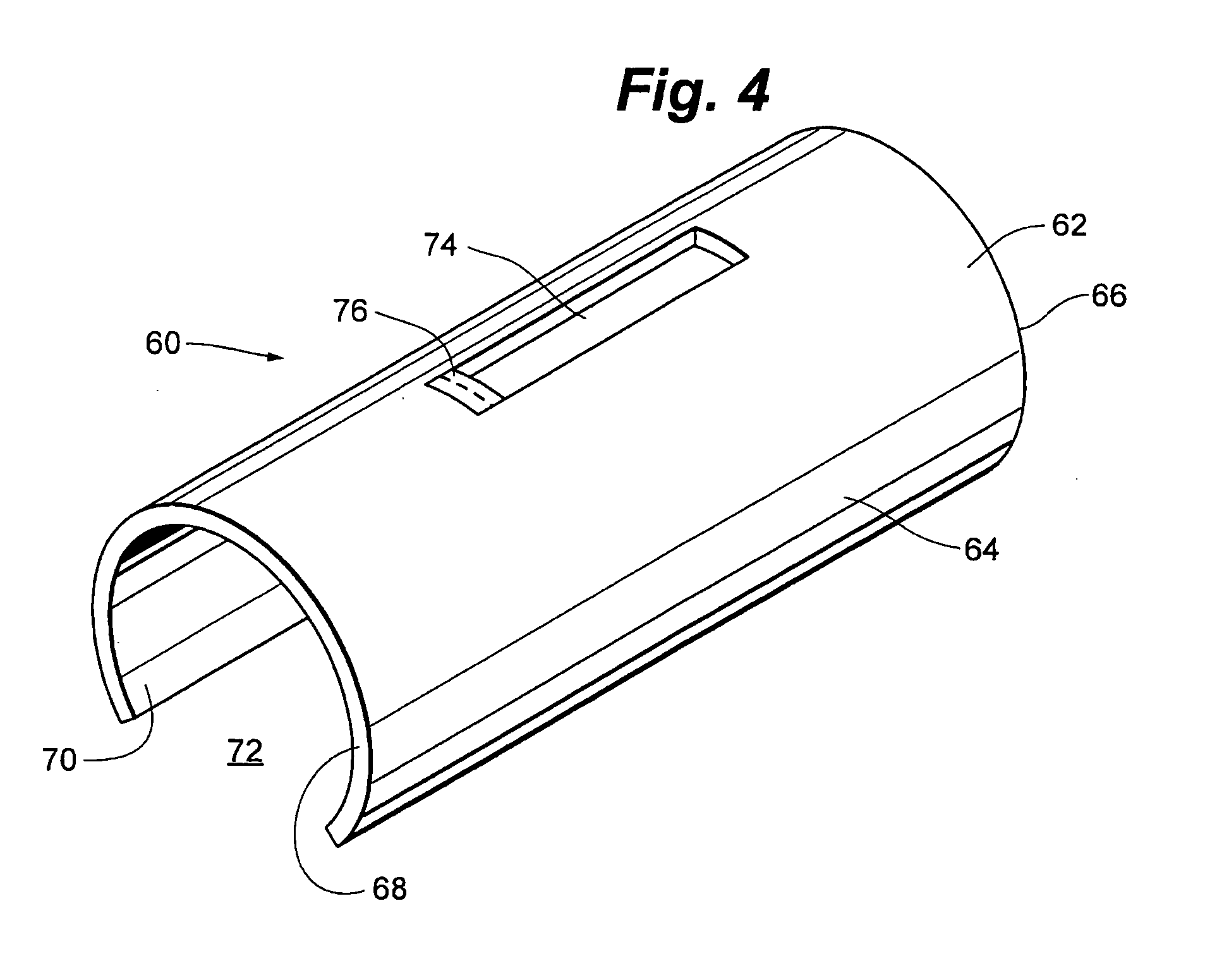
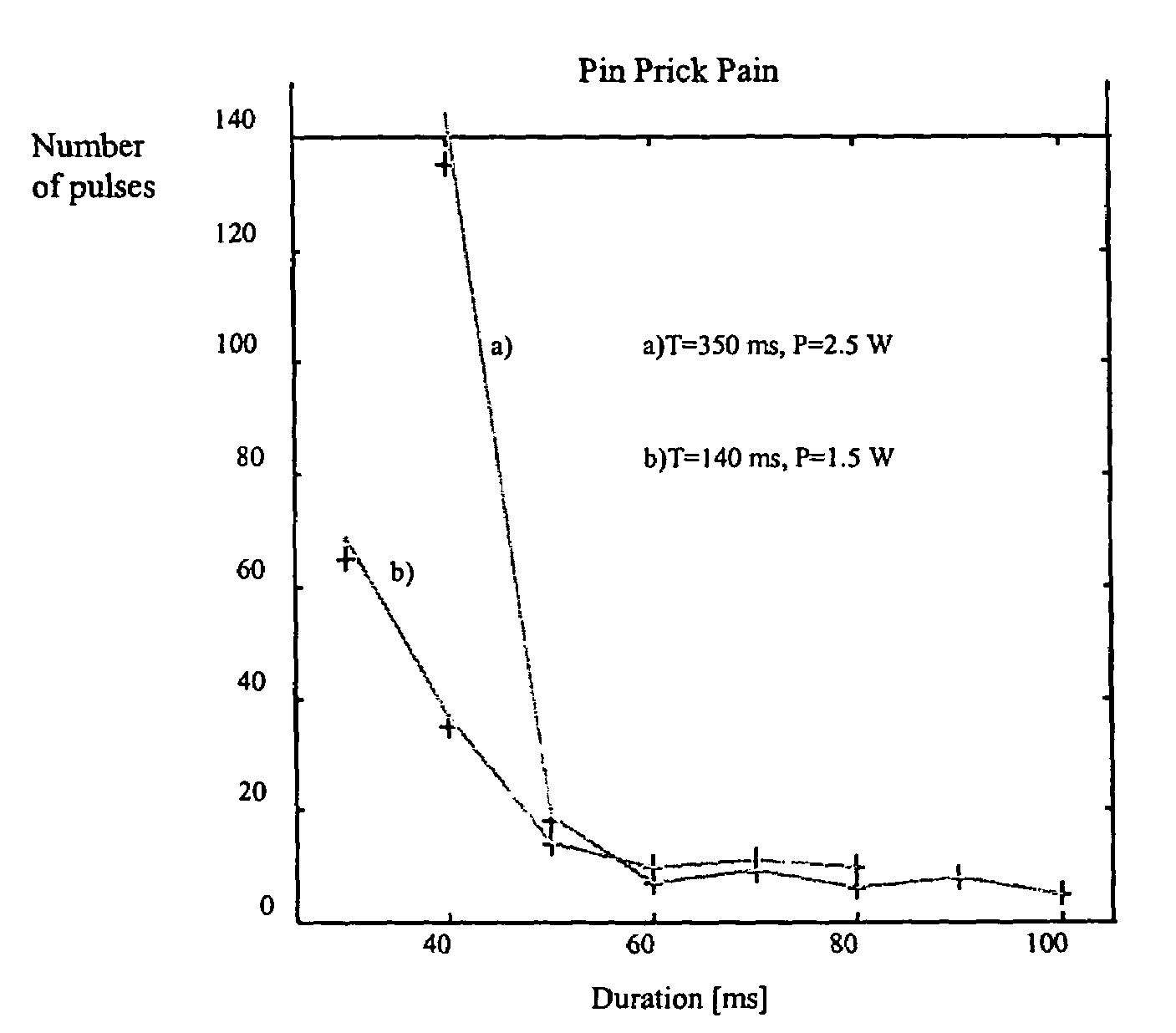
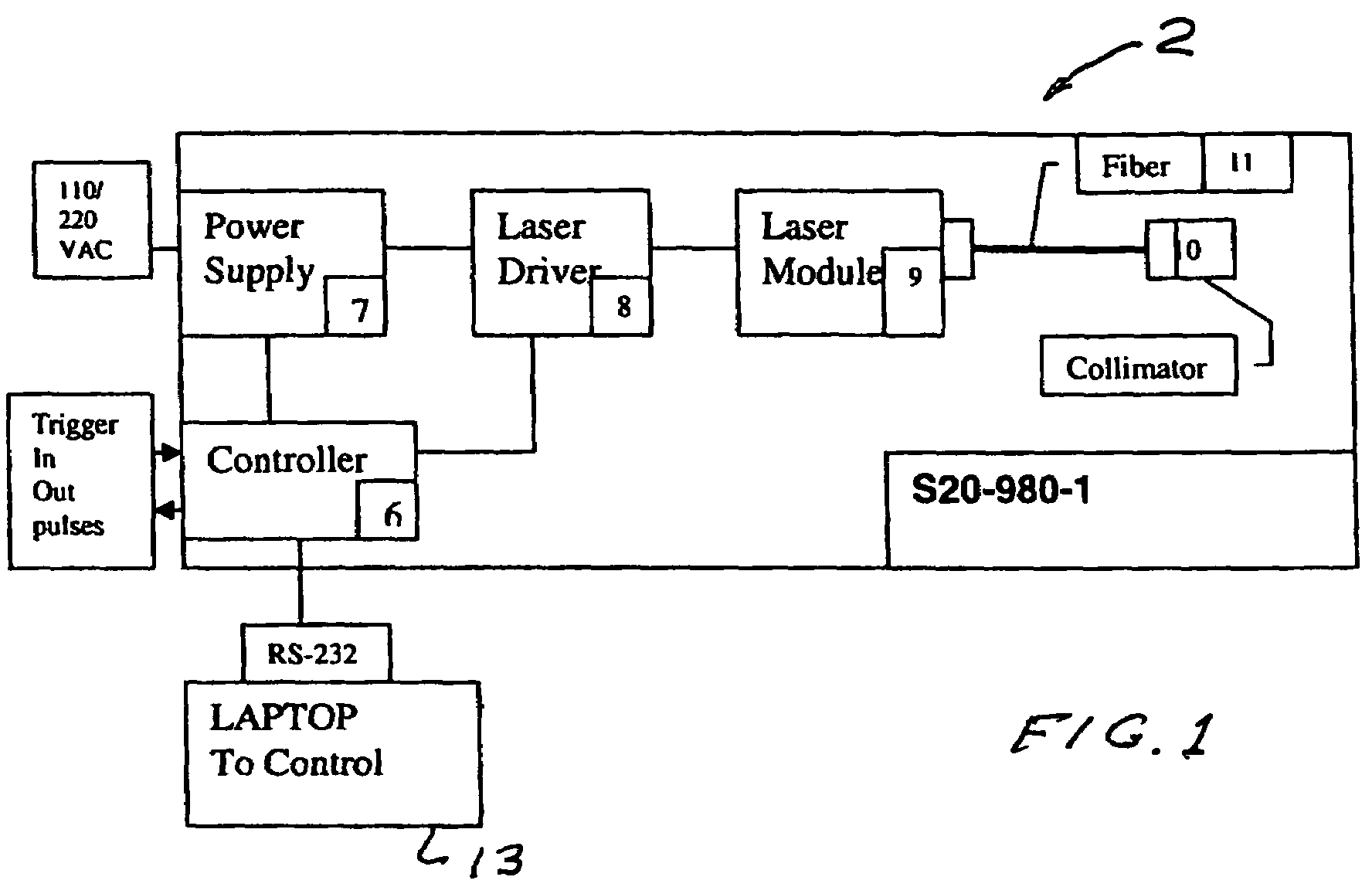
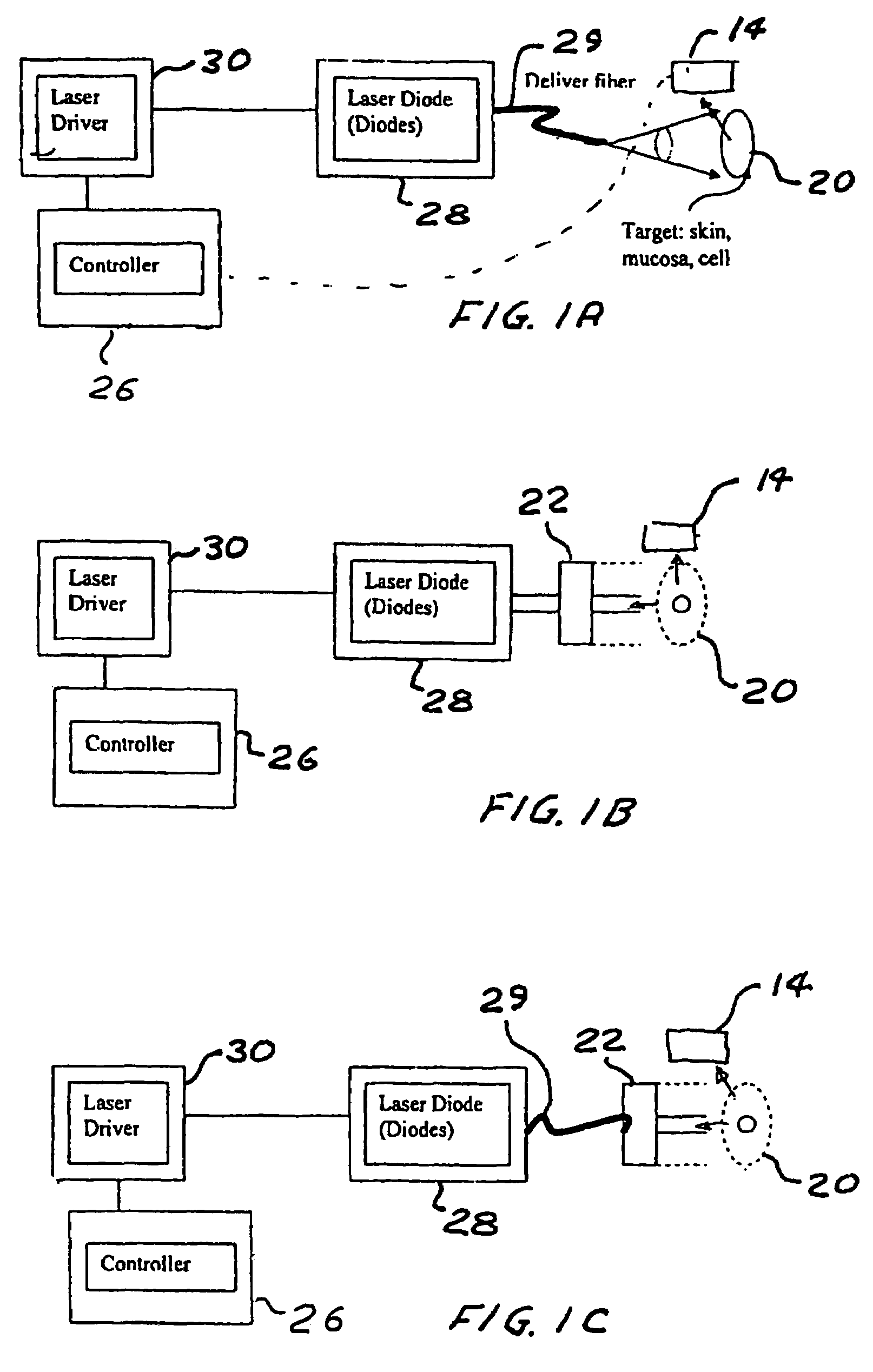
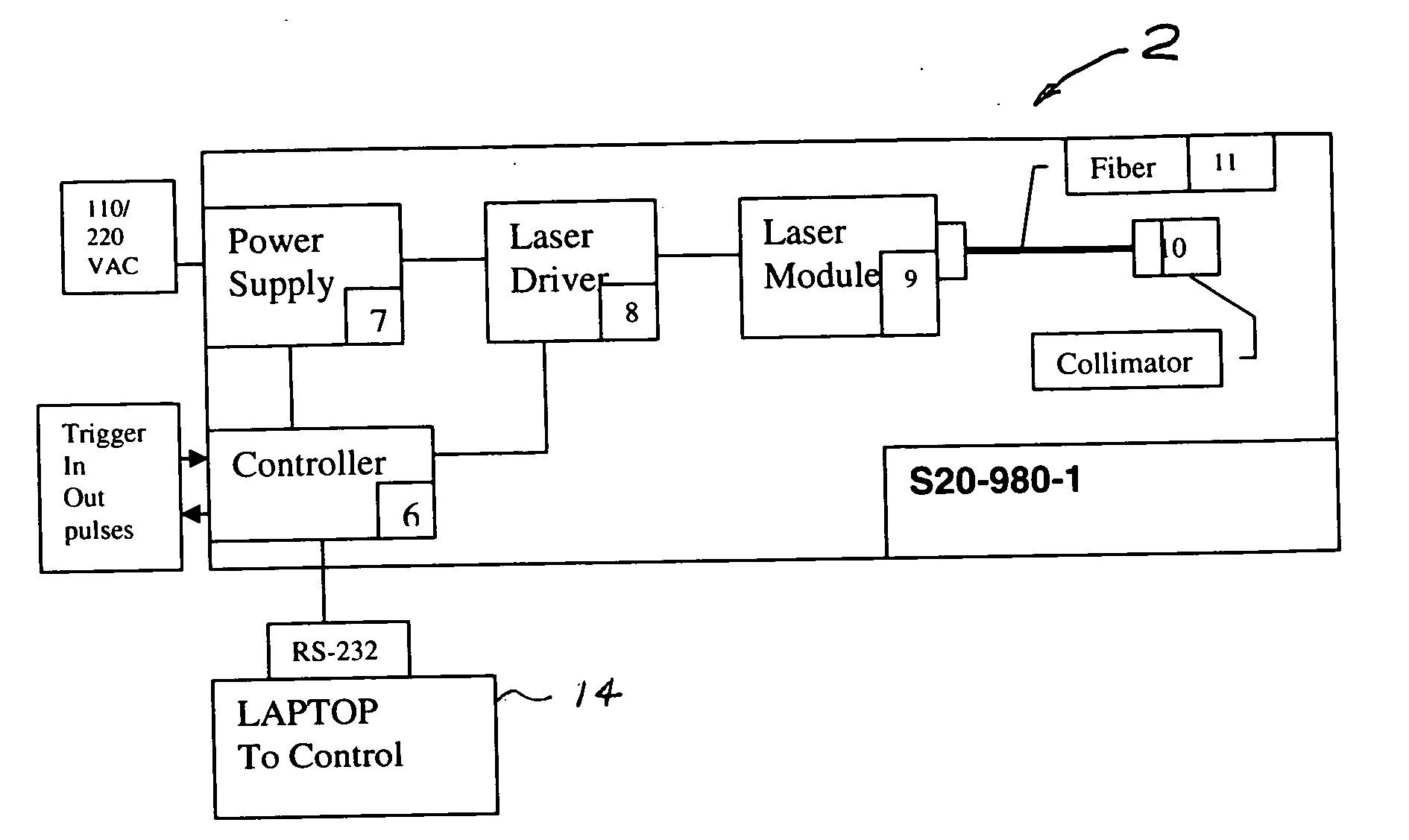
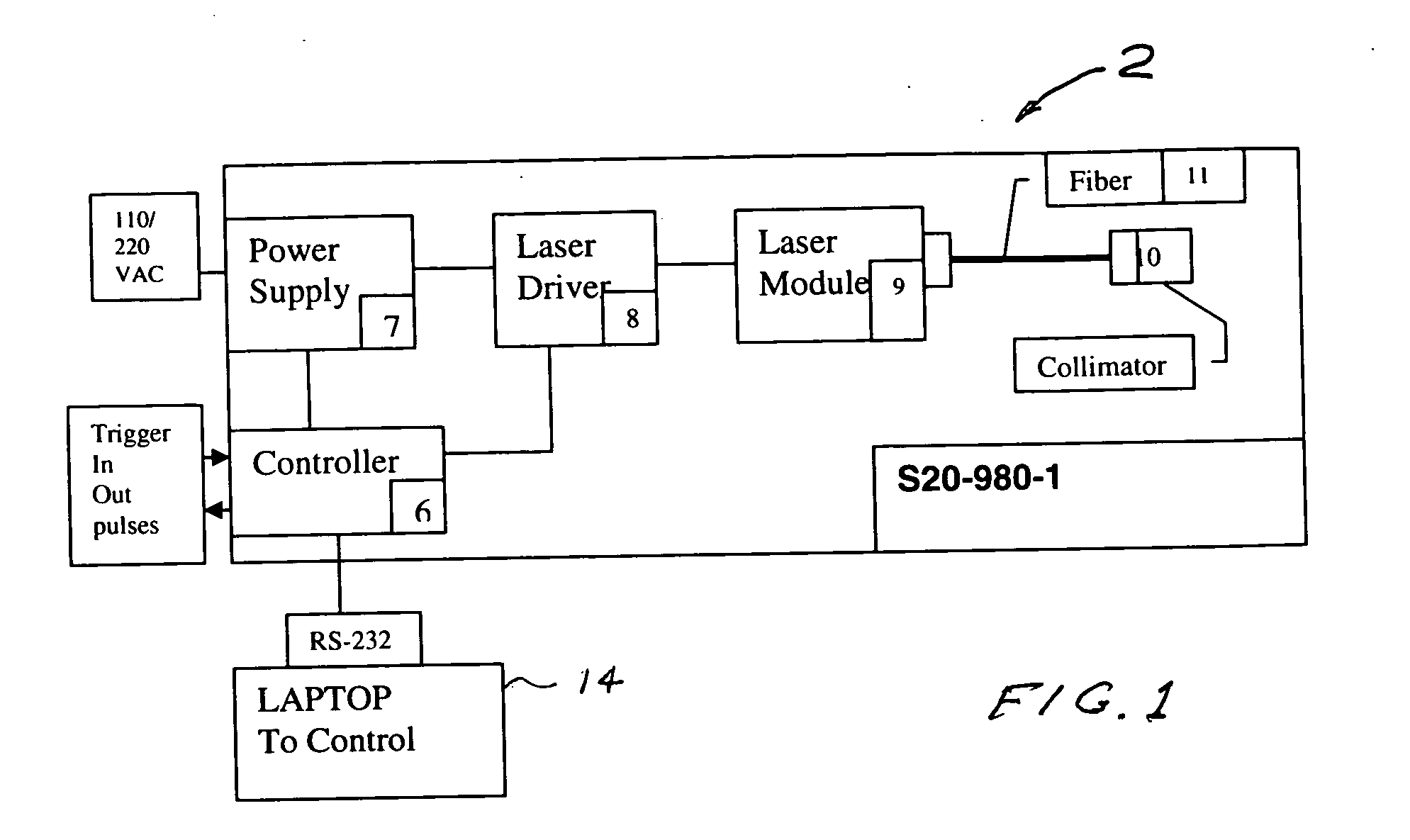
Portable laser and process for producing controlled pain
A process and laser system for in vitro and in vivo pain research, pain clinical testing and pain management. In preferred embodiments of the present invention a diode laser operating at a 980 nm wavelength is used to produce warmth, tickling, itching, touch, burning, hot pain or pin-prick pain. The device and methods can be used for stimulation of a single nerve fiber, groups of nerve fibers, nerve fibers of single type only as well as more the one type of nerve fibers simultaneously. The present invention is especially useful for research of human / animal sensitivity, pain management, drug investigation and testing, and psychophysiology / electrophysiology studies. The device and methods permit non-contact, reproducible and controlled tests that avoid risk of skin damage. Applicant an his fellow workers have shown that tests with human subjects with the process and laser of the present invention correlate perfectly with laboratory tests with nerve fibers of rats. The device and the methods can be applied in a wide variety of situations involving the study and treatment of pain. Preferred embodiments of the present invention provide laser systems and techniques that permit mapping and single mode activation of C fibers and A-delta fibers.
Owner:NEMENOV MIKHAIL
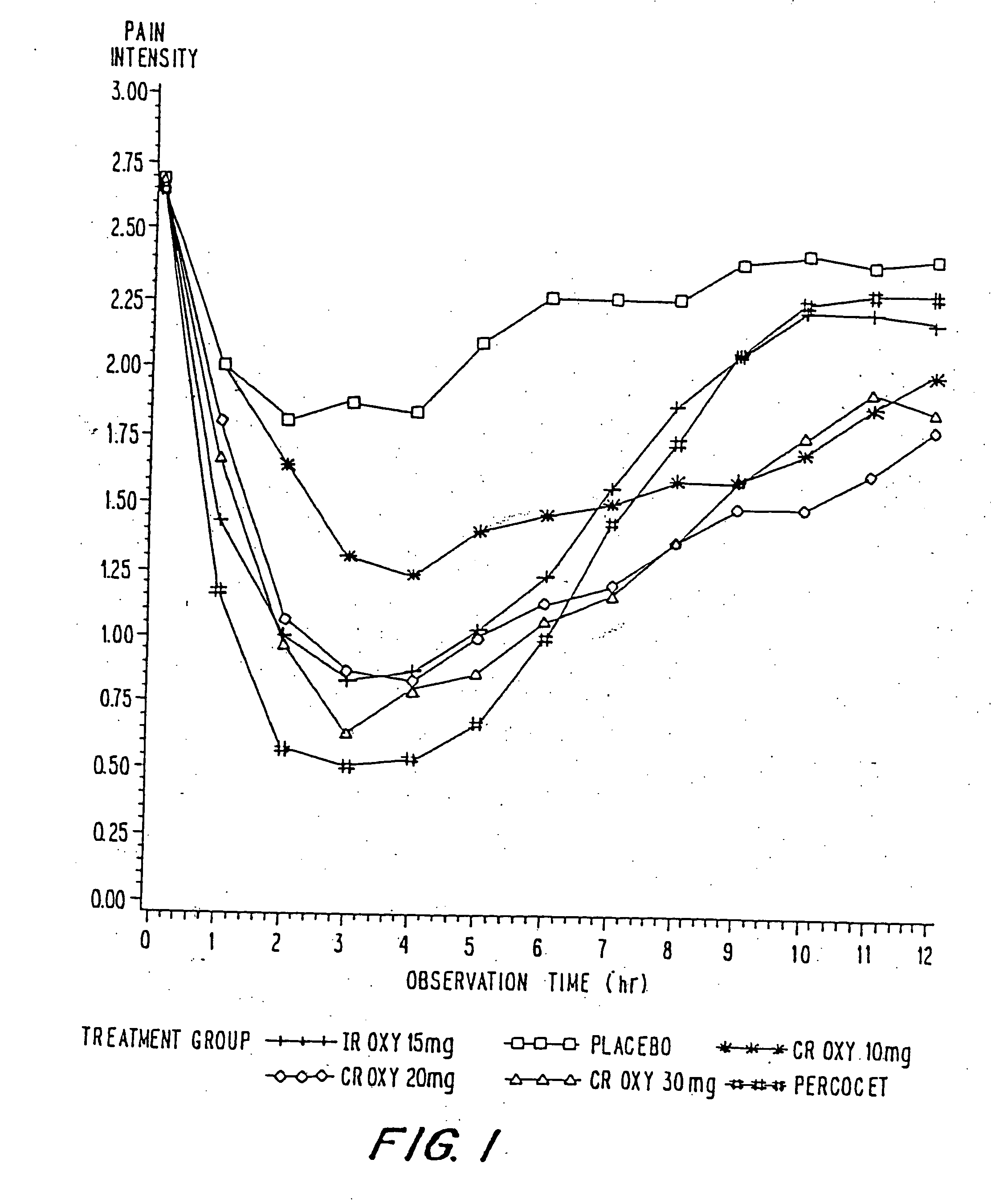
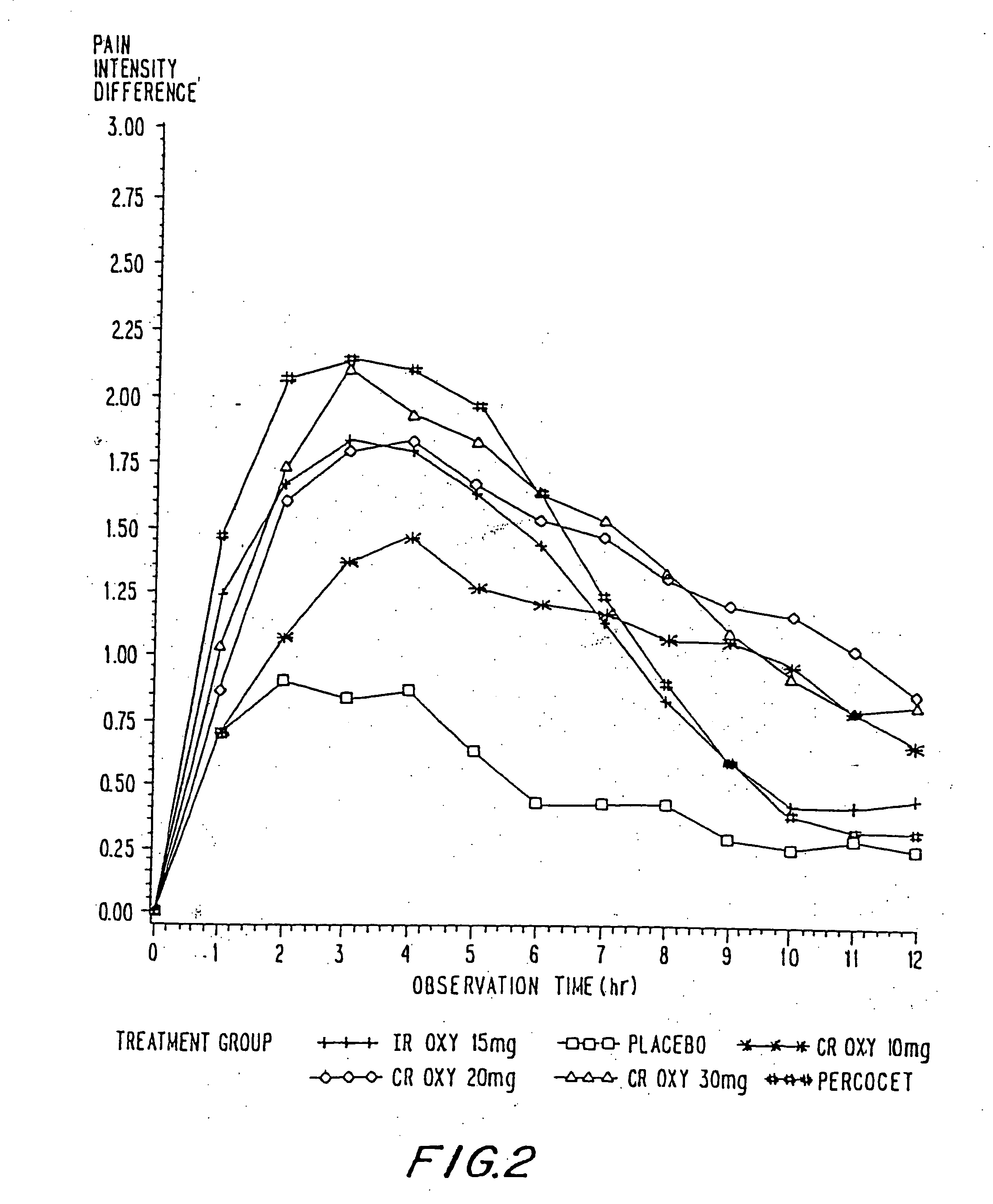
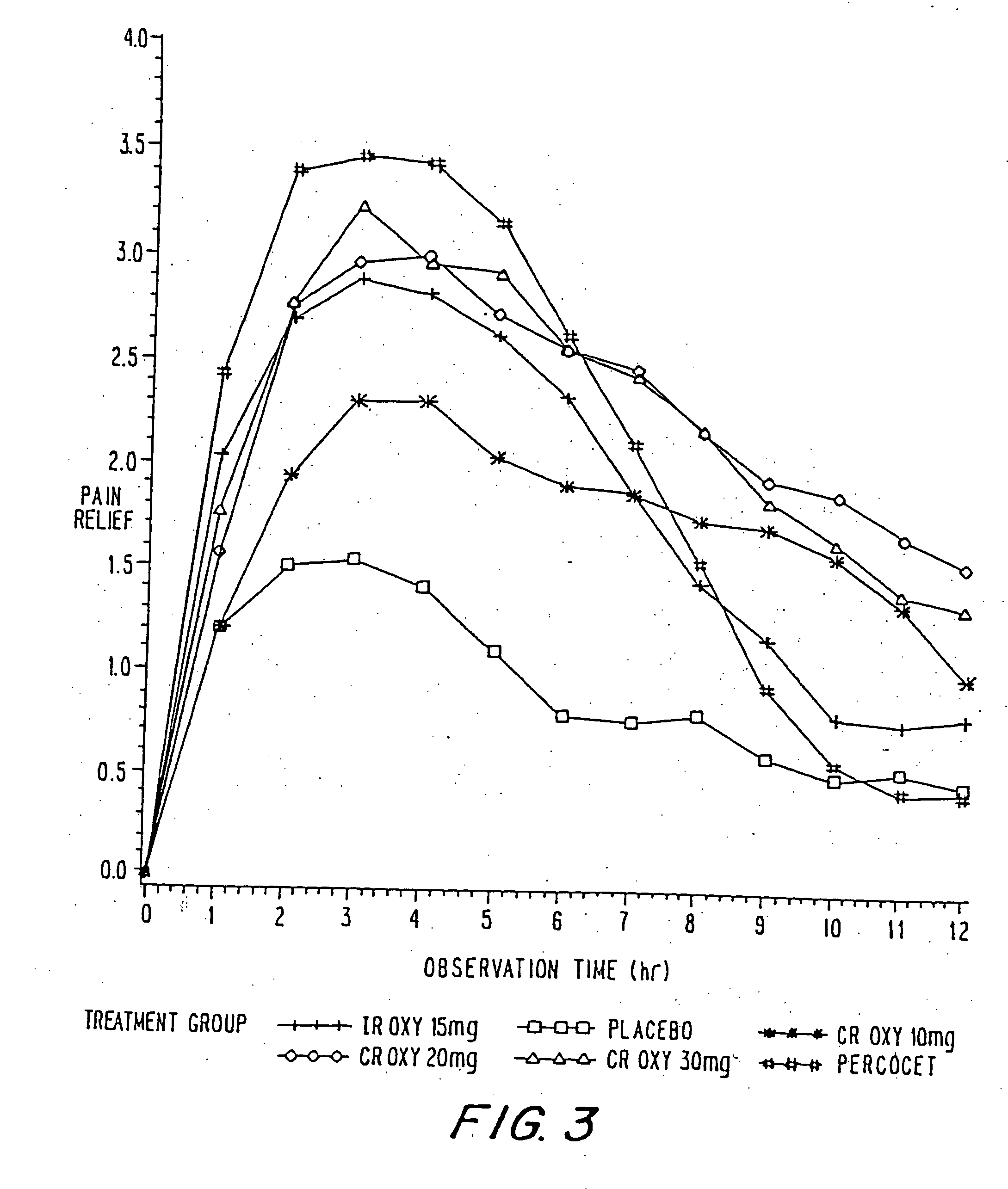
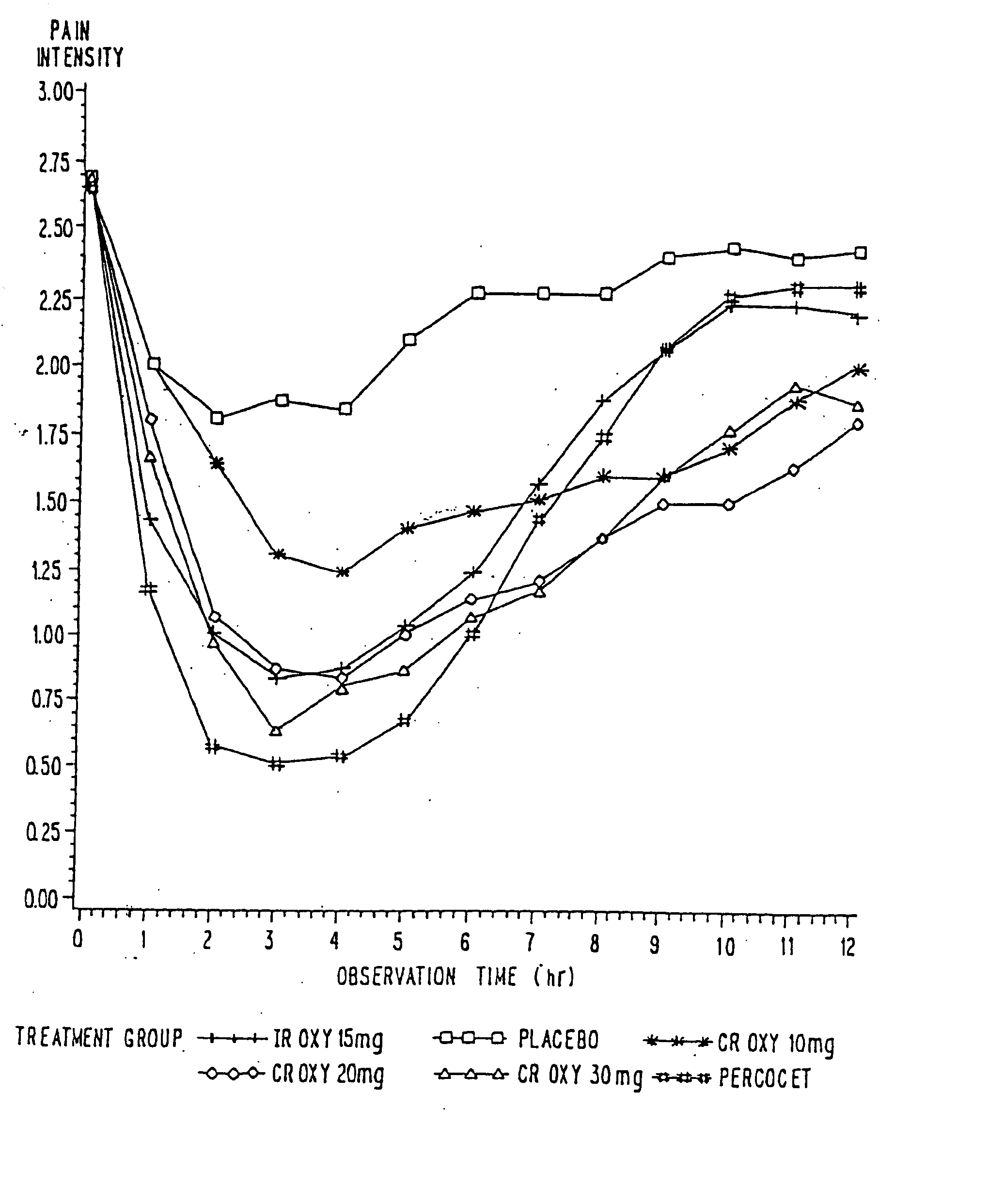
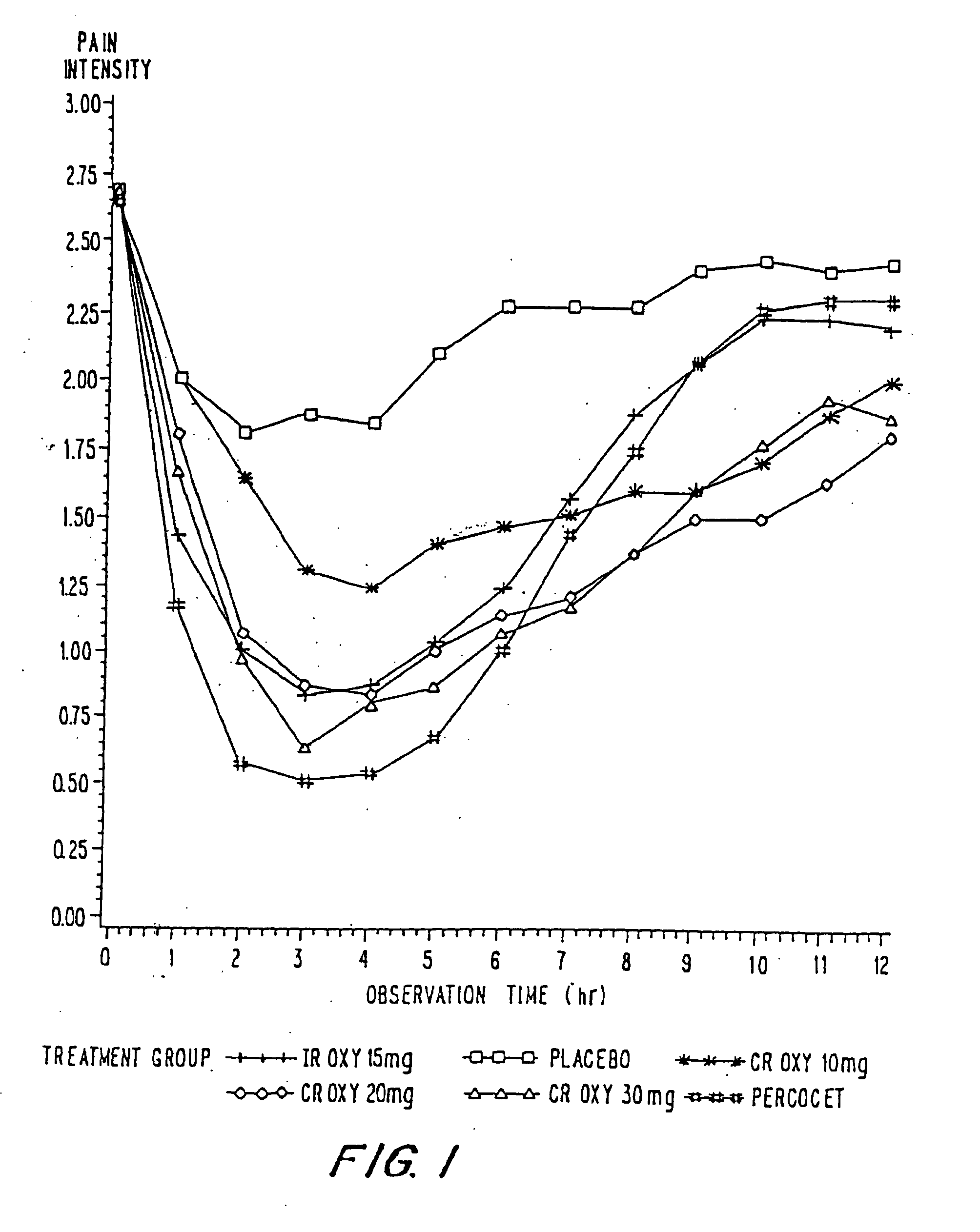
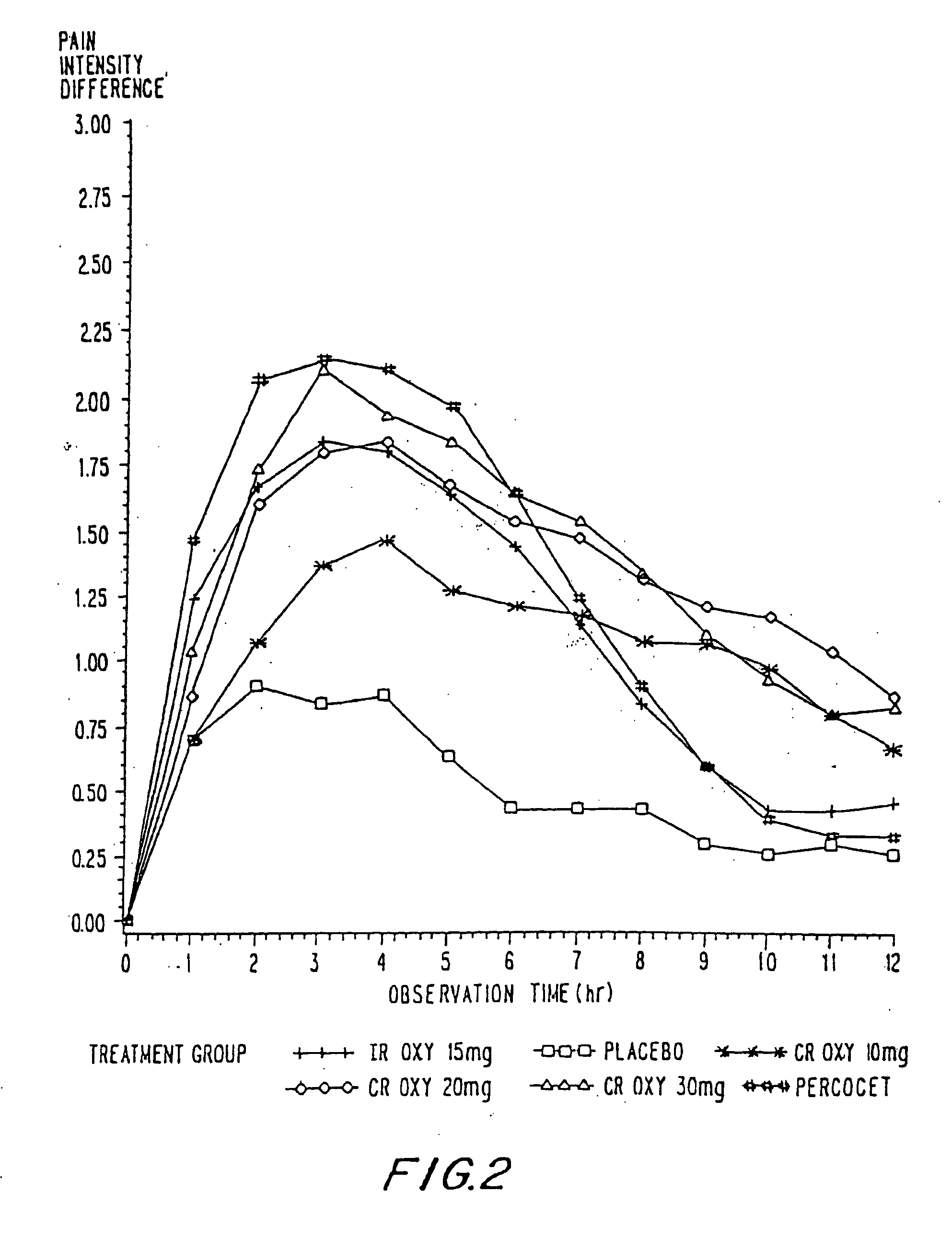
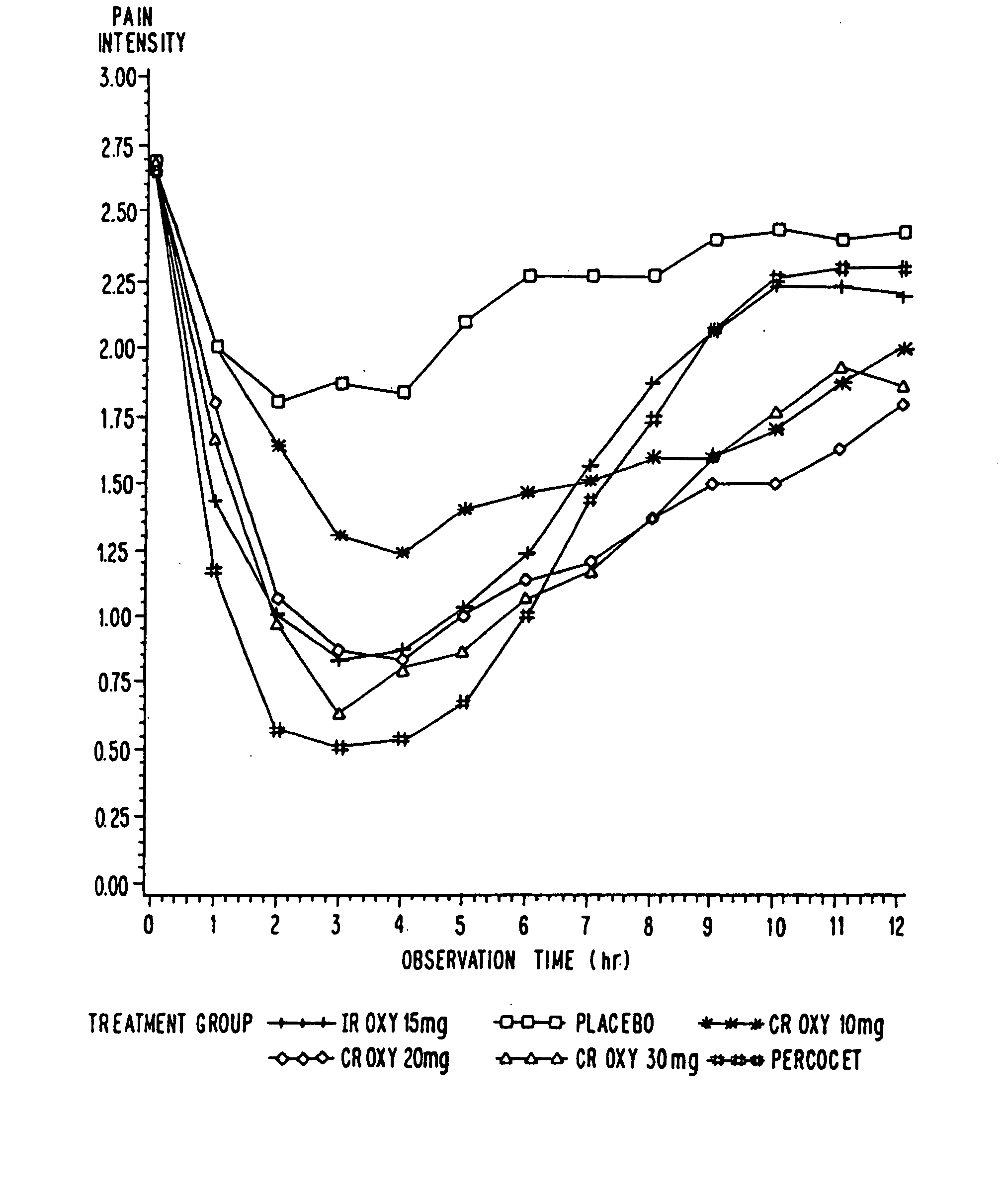
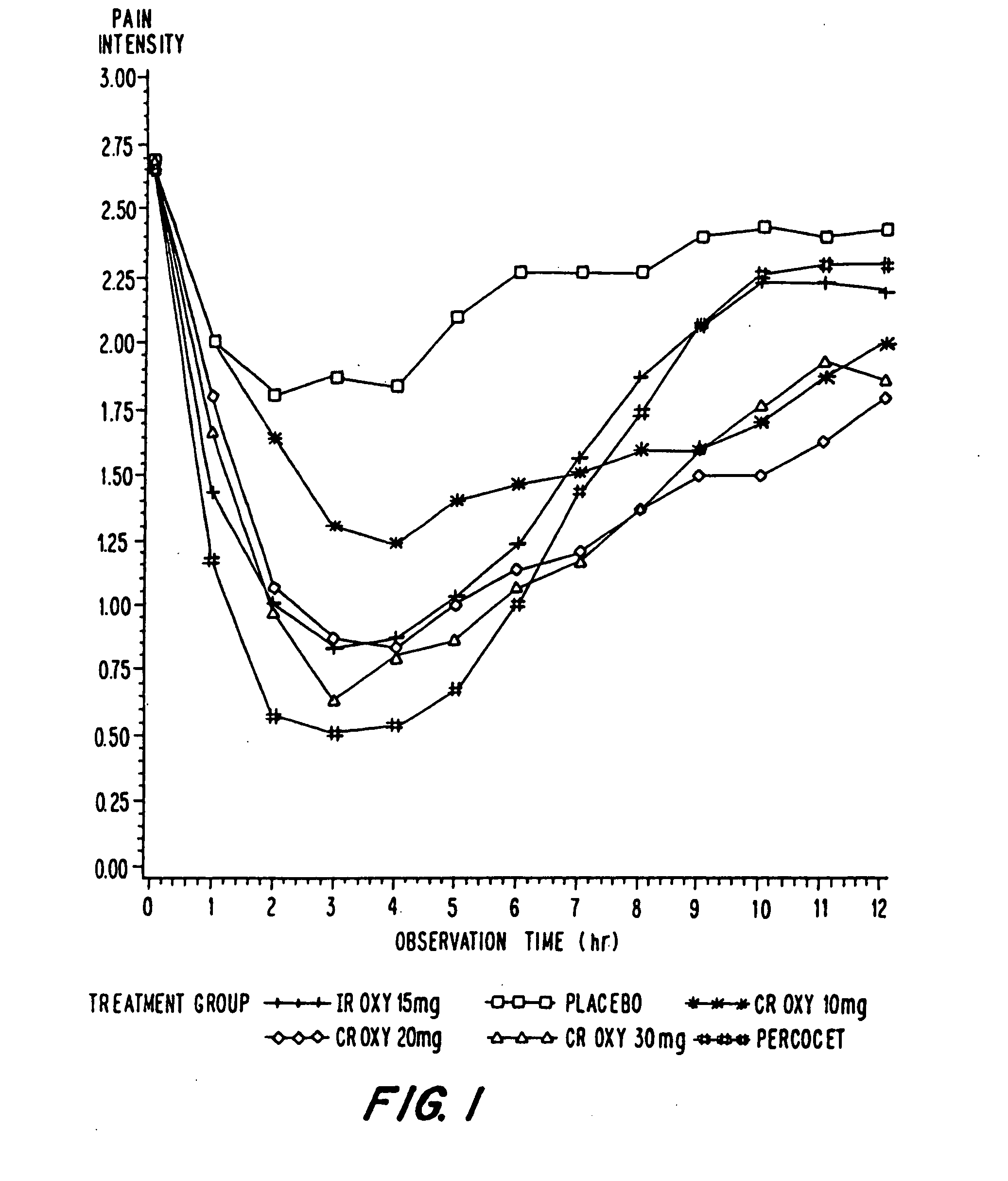
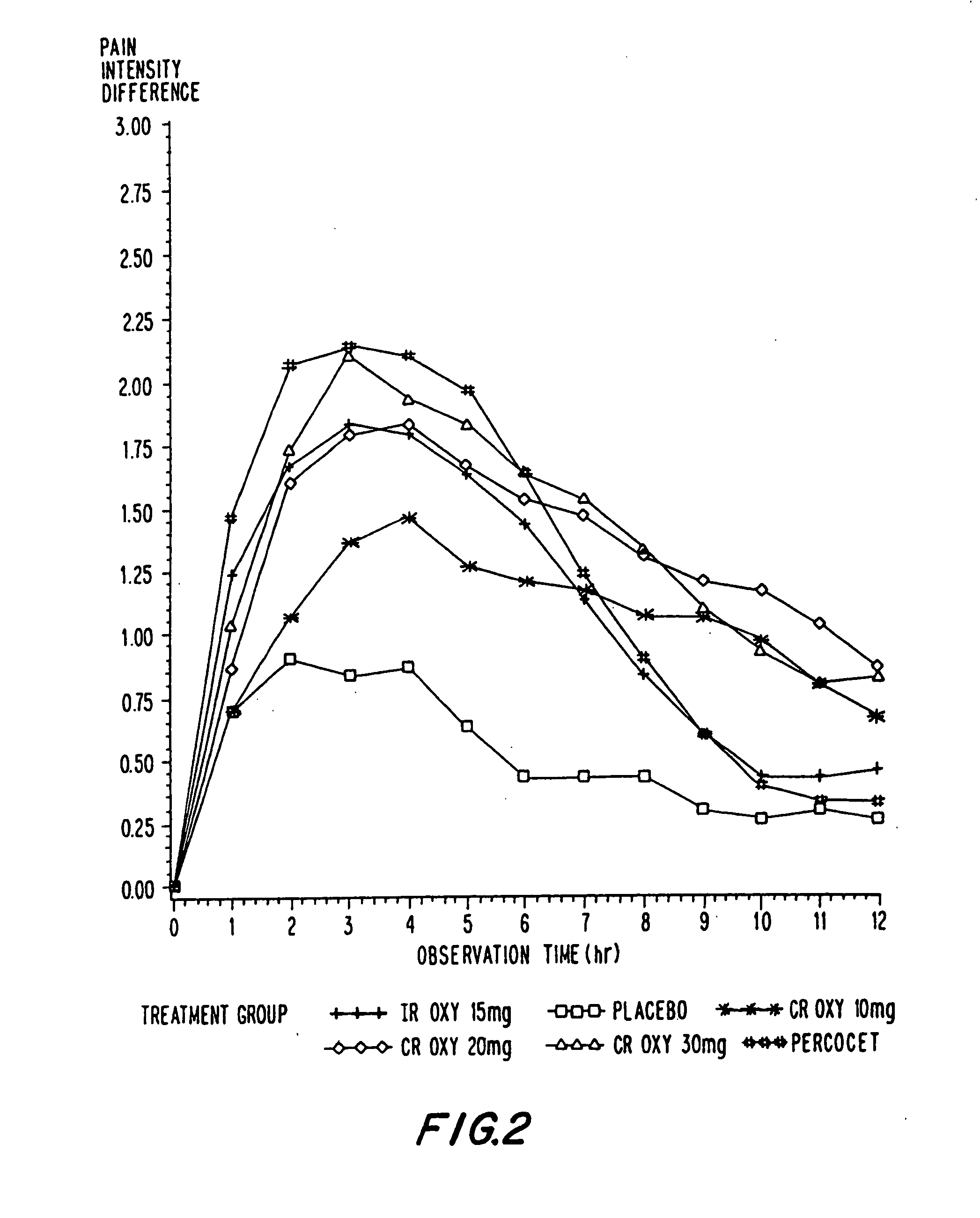
Controlled release oxycodone compositions
InactiveUS20060057210A1Improve efficiencyQuality improvementBiocidePowder deliveryControlled releaseBlood plasma
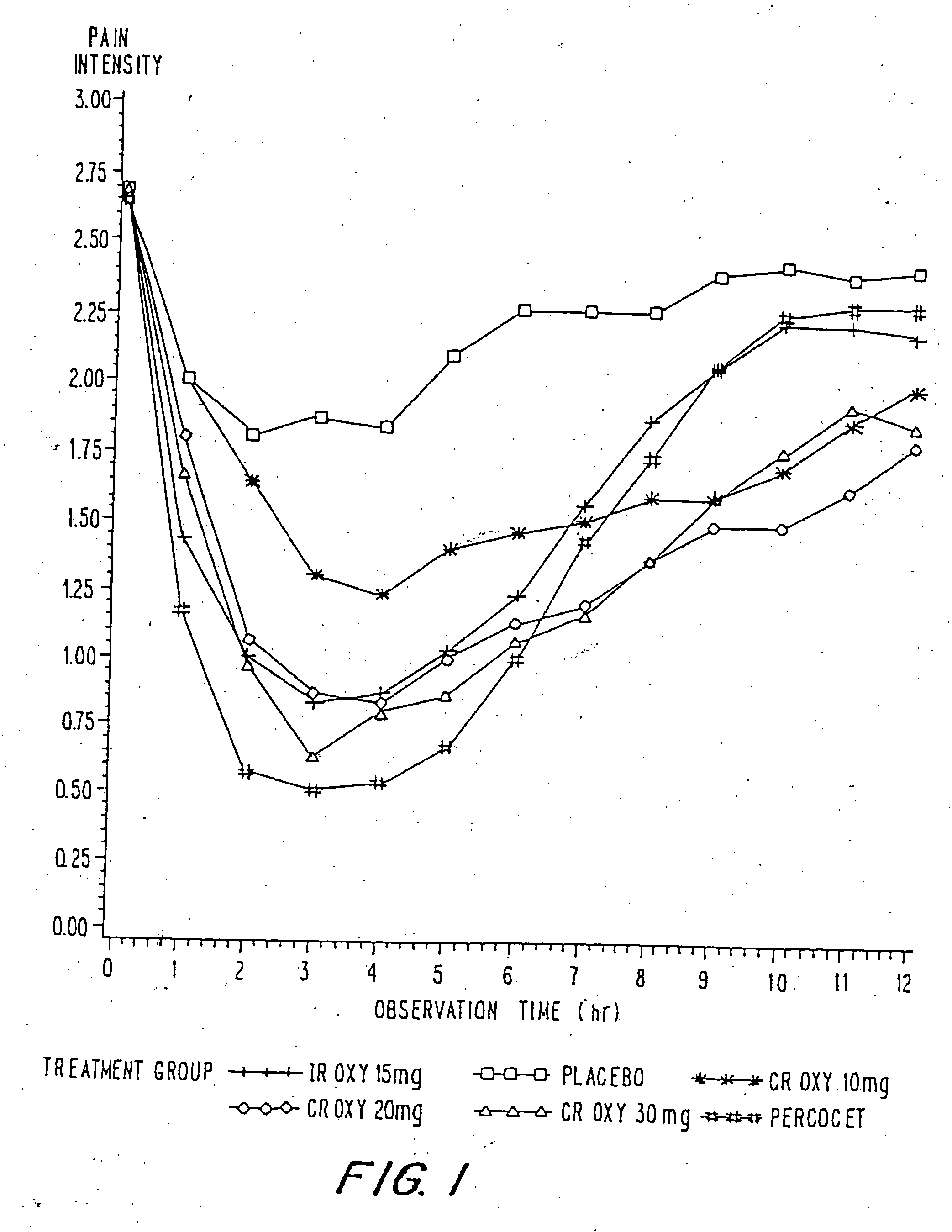
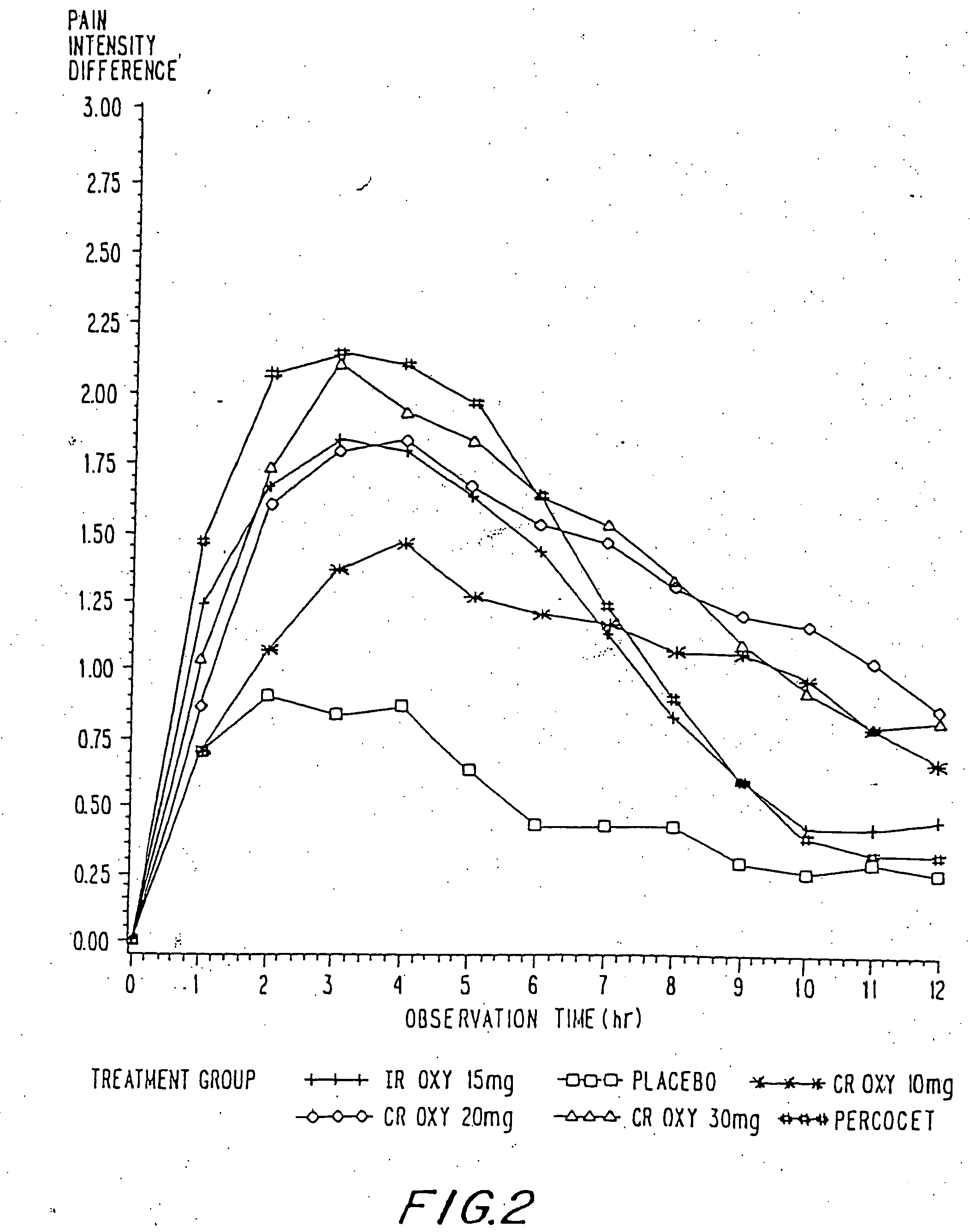
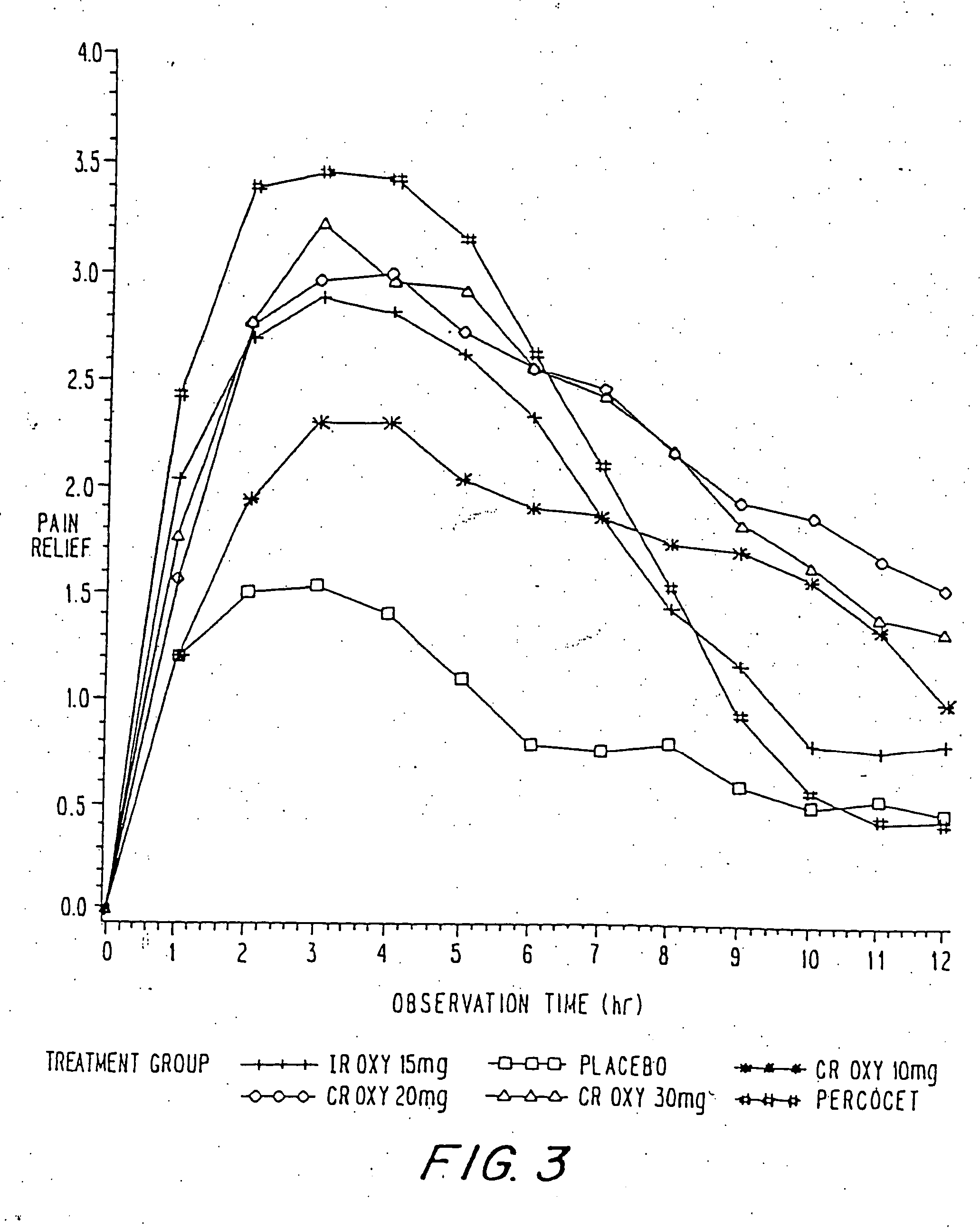
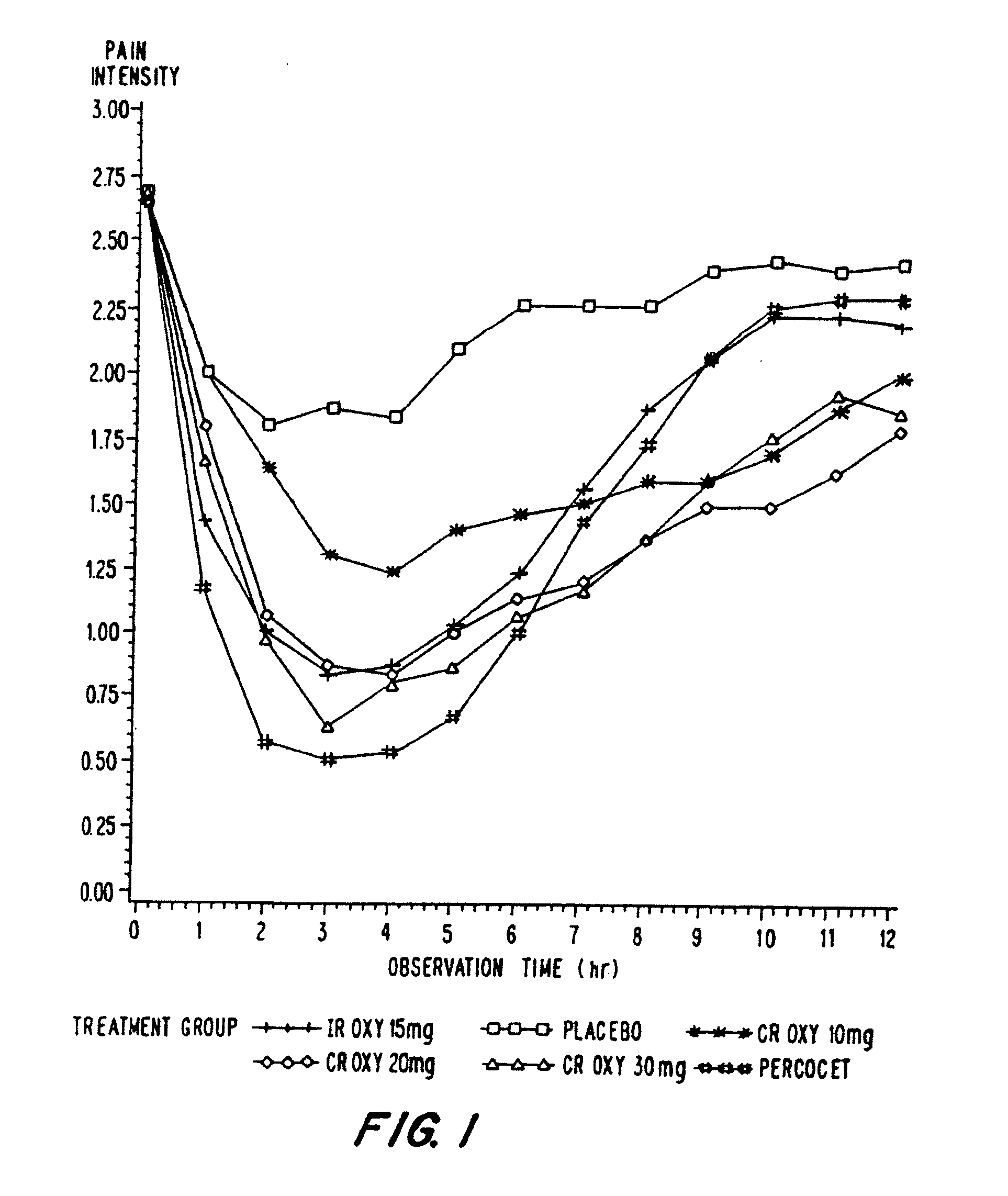
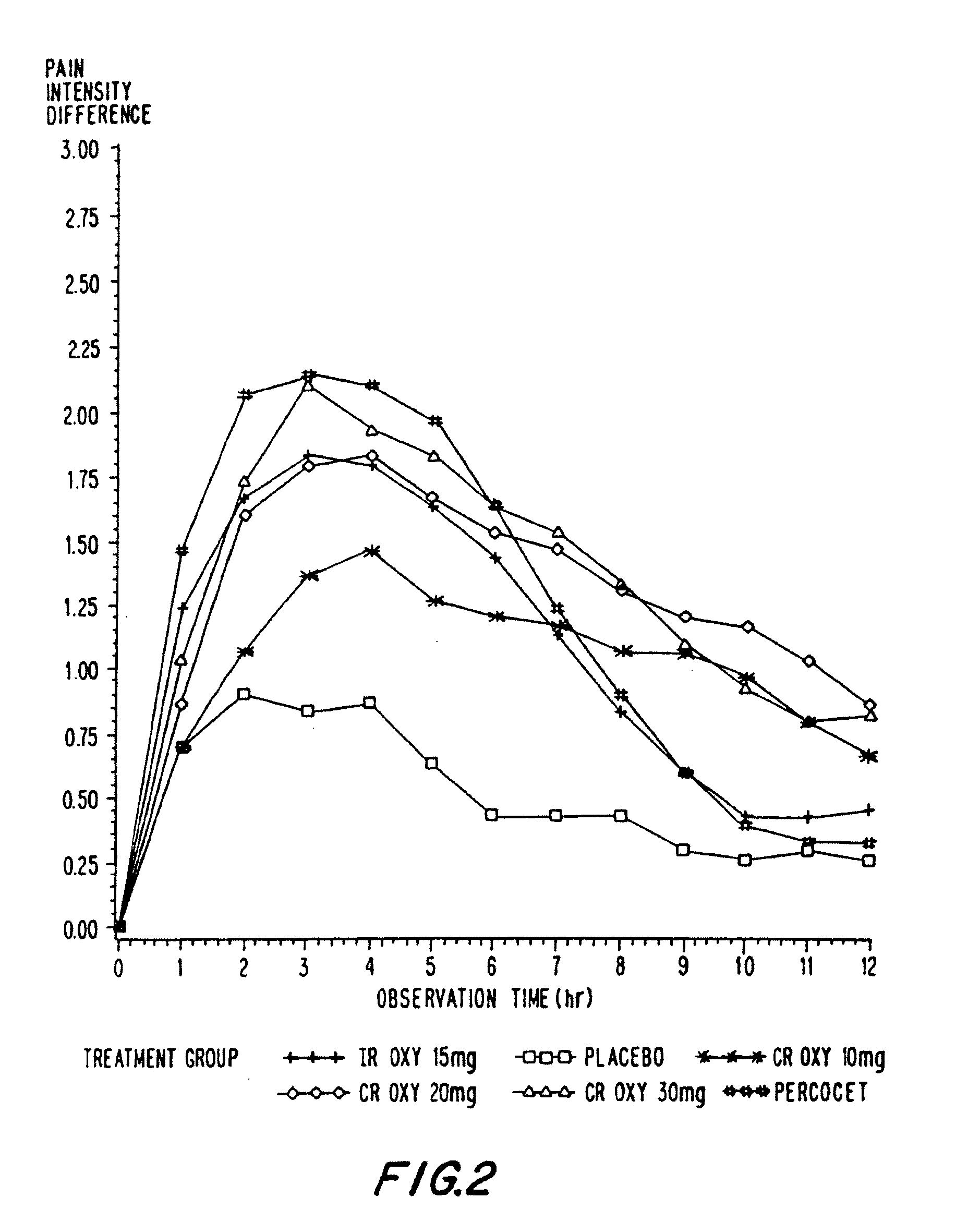
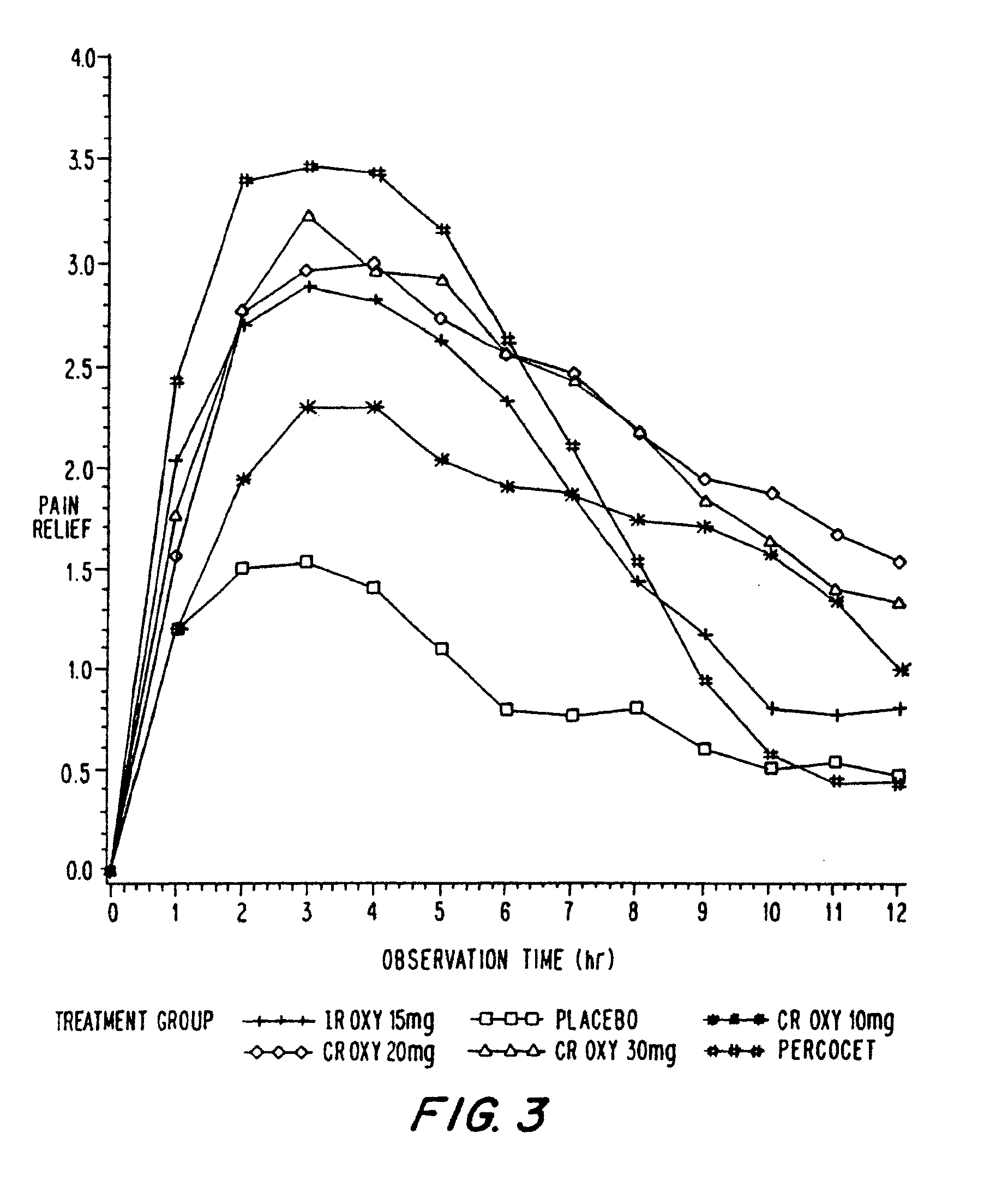
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +2
Controlled release oxycodone compositions
InactiveUS20060099255A1Improve efficiency and qualityOrganic active ingredientsNervous disorderControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:OSHLACK BENJAMIN +3
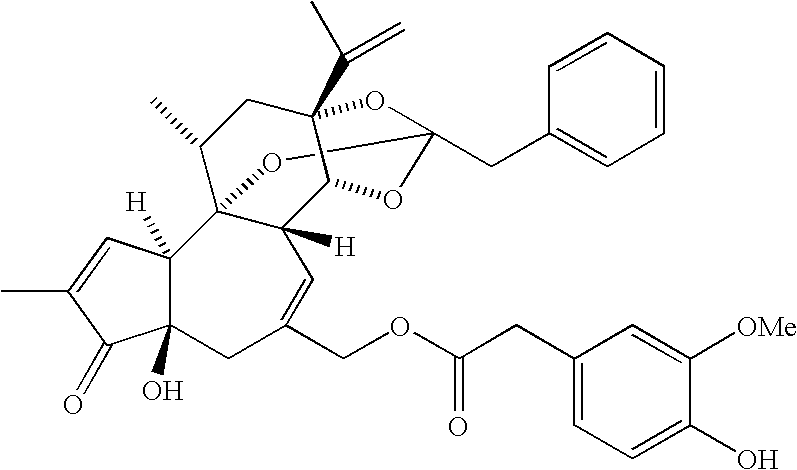
Fused compounds that inhibit vanilloid receptor subtype 1 (VR1) receptor
The present invention discloses novel compounds of general formula (I) or a pharmaceutically acceptable salt or prodrug thereof (in which X1—X5, R5—R8b, Z1-Z2 and Ar1 are defined herein), a method for inhibiting the VR1 receptor in mammals using these compounds, a method for controlling pain in mammals, and pharmaceutical compositions including those compounds and a process for making those compounds.
Owner:ABBVIE INC
Method and apparatus for multimodal electrical modulation of pain using composite electromagnetic fields
ActiveUS20180028812A1Chronic painGood pain reliefSpinal electrodesExternal electrodesVariable CharacteristicElectromagnetic field
Apparatus and methods for managing pain uses a single composite modulation / stimulation signal with variable characteristics to achieve the same results as separate varying electromagnetic signals. The composite signal is utilized for modulating the expression of genes involved in diverse pathways including inflammatory / immune system mediators, ion channels and neurotransmitters, in both the Spinal Cord (SC) and Dorsal Root Ganglion (DRG) where such expression modulation is caused by spinal cord stimulation or peripheral nerve stimulation using the disclosed apparatus and techniques.
Owner:MEDTRONIC SG LLC
Controlled release oxycodone compositions
InactiveUS20100092570A1Improve efficiency and qualityLow variabilityBiocidePowder deliveryControl releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +1
Method of inducing topical anesthesia and transdermal patch
InactiveUS20090123527A1Avoid the needLower potentialPowder deliveryBiocideTransdermal patchPhysiological fluid
Disclosed is a method of inducing topical anesthesia in a tissue or organ of an animal comprising providing an aqueous gel formulation comprising water, an anesthetic (e.g., lidocaine hydrochloride), a viscoelastic polymer, and a tonicity modifier, wherein the aqueous gel formulation is free of preservatives and phosphate buffer, is isotonic with physiological fluids, and is sterile and has low particulate count. Also disclosed are a transdermal patch comprising the aqueous gel formulation suitable for applying on the skin of a patient and a method of controlling pain therewith.
Owner:AKORN
Controlled release oxycodone compositions
InactiveUS20100034876A1Improve efficiency and qualityBiocidePowder deliveryControlled releaseMedicine
Owner:PURDUE PHARMA LP +1
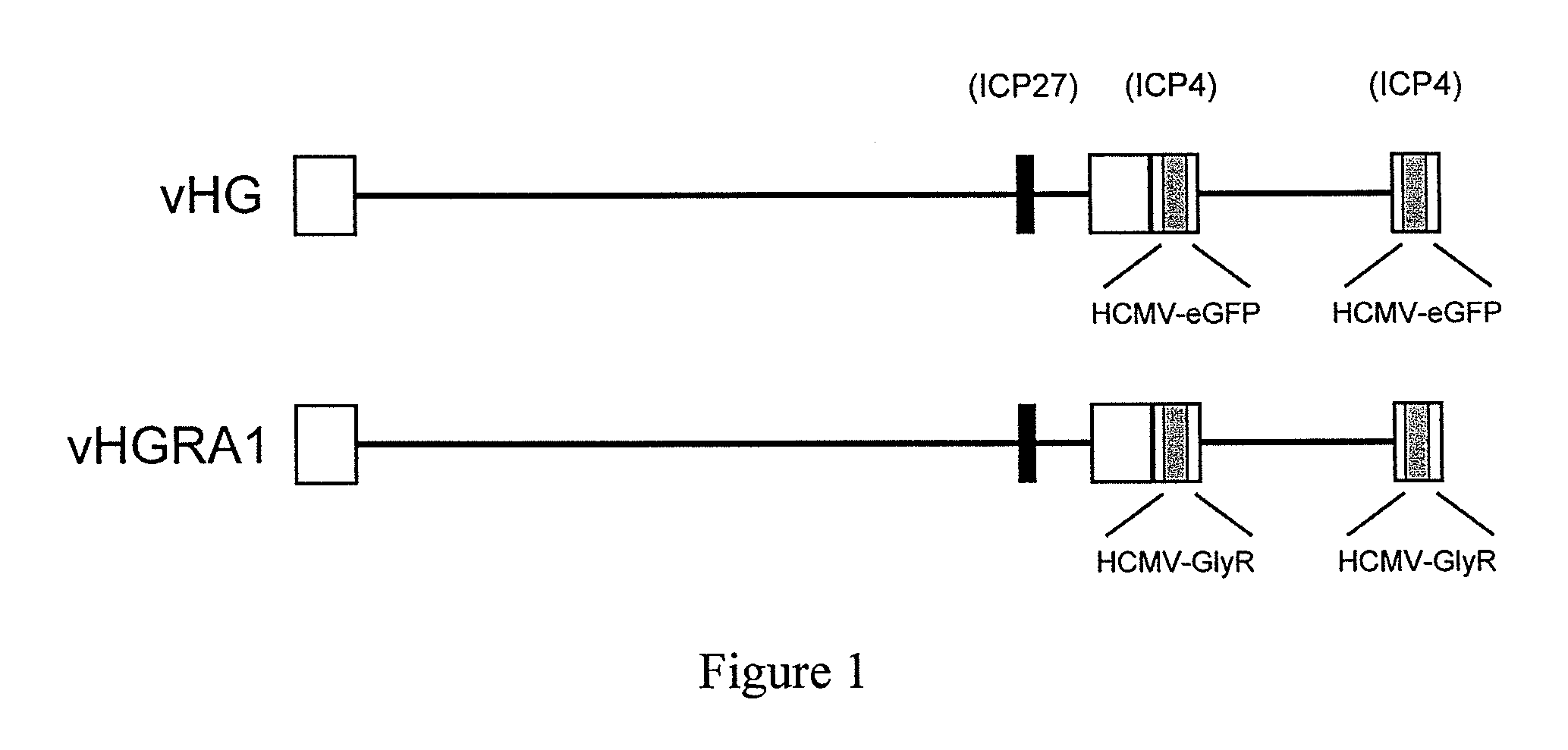
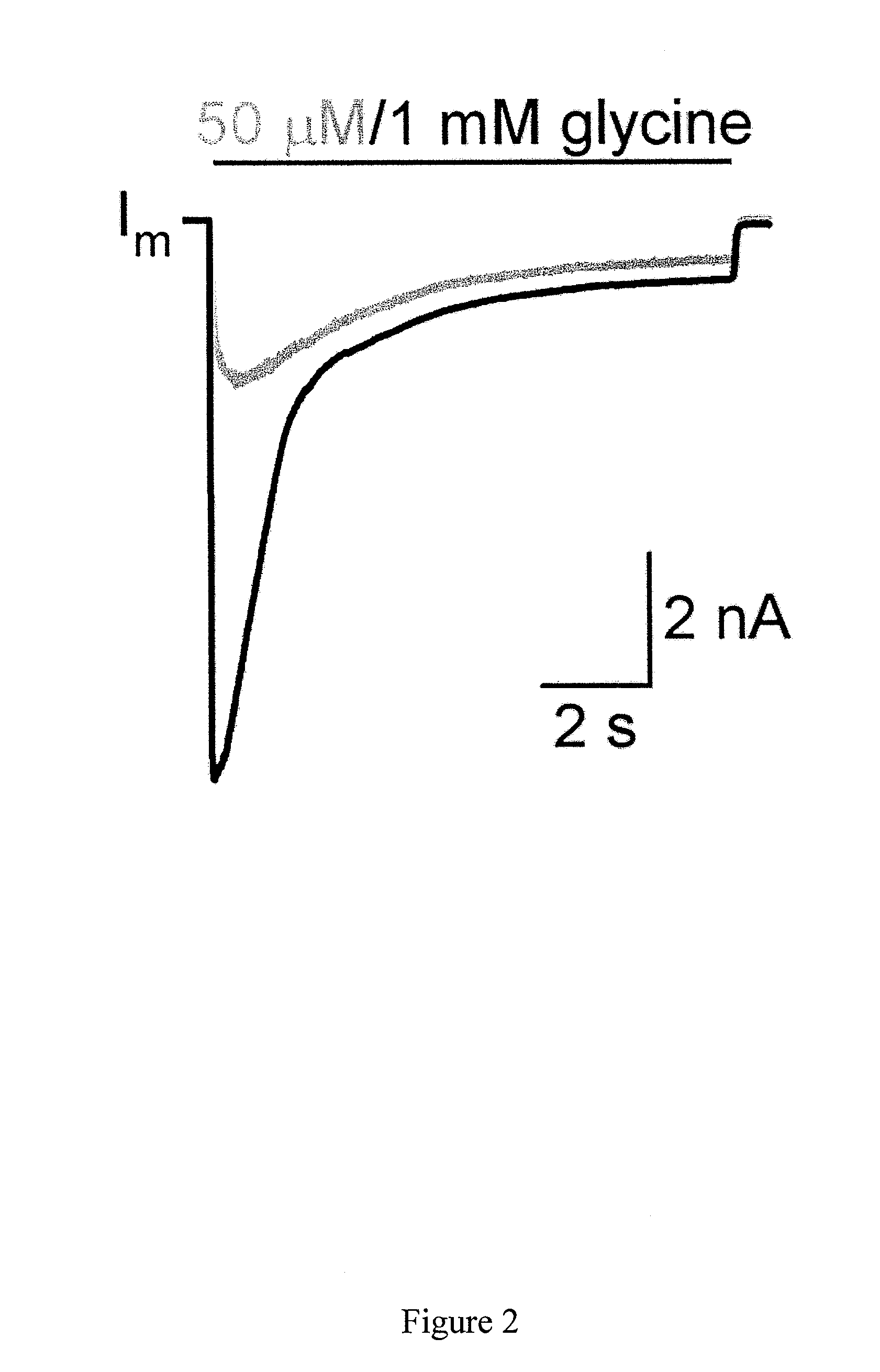
Targeted delivery of glycine receptors to excitable cells
InactiveUS20080289058A1Reduce decreaseReduces formalin-induced nociceptive behaviorOrganic active ingredientsNervous disorderGlycine receptorAllosteric modulator
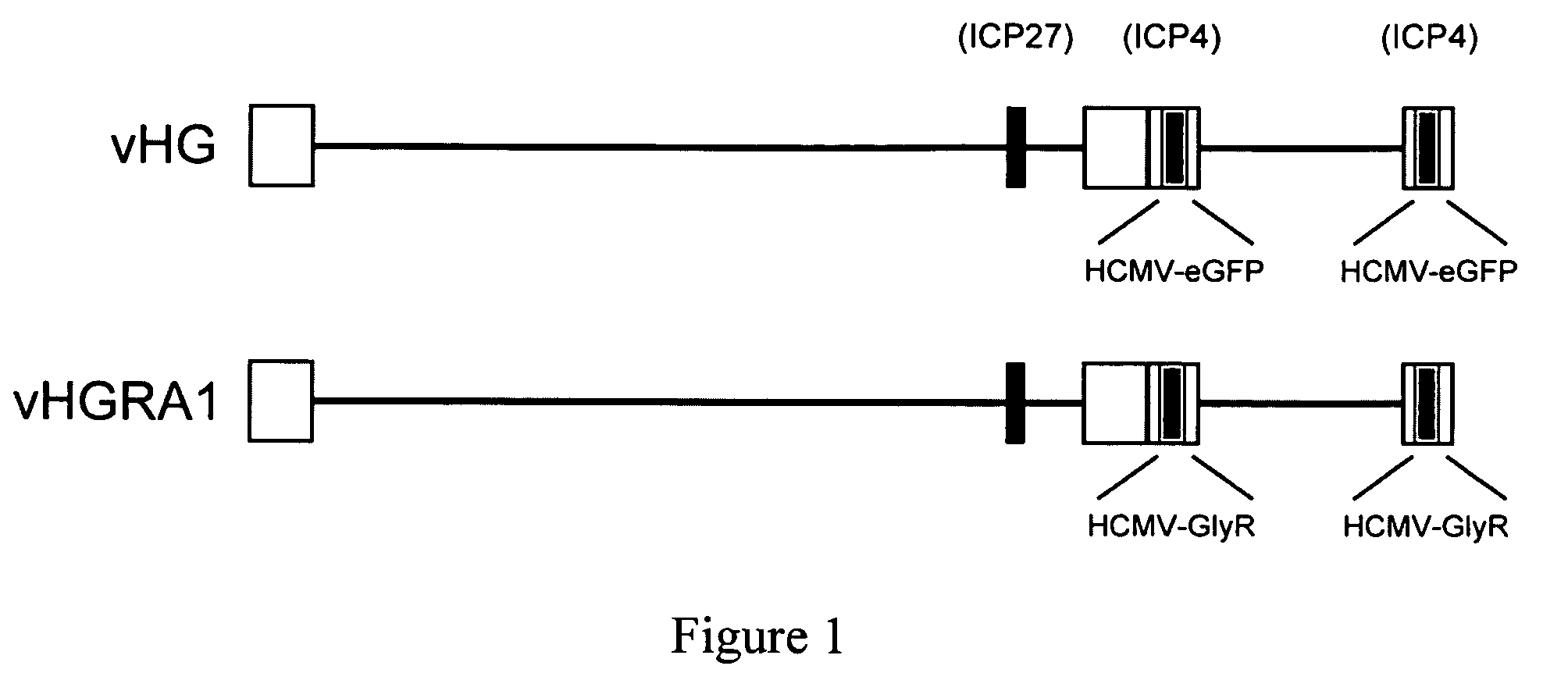
The invention provides a method of modulating electrophysiological activity of an excitable cell. The method involves causing exogenous expression of a glycine receptor (GlyR) protein in an excitable cell of a subject. Thereafter, the excitable cell is exposed to an allosteric modulator of the GlyR protein. Modulation of the exogenous GlyR protein (an ion channel) in response to the allosteric modulator modulates the electrophysiological activity of the excitable cell. The method can be used to control pain in a subject. The invention further provides a replication-defective HSV vector comprising an expression cassette encoding a GlyR protein, stocks and pharmaceutical compositions containing such vectors, and a transgenic animal.
Owner:UNIVERSITY OF PITTSBURGH
Selective ablation of pain-sensing neurons by administration of a vanilloid receptor agonist
The present invention provides methods and kits for the selective ablation of pain-sensing neurons. The methods comprise administration of a vanilloid receptor agonist to a ganglion in an amount that causes death of vanilloid receptor-bearing neurons. Accordingly, the present invention provides methods of controlling pain and inflammatory disorders that involve activation of vanilloid receptor-bearing neurons.
Owner:UNITED STATES OF AMERICA
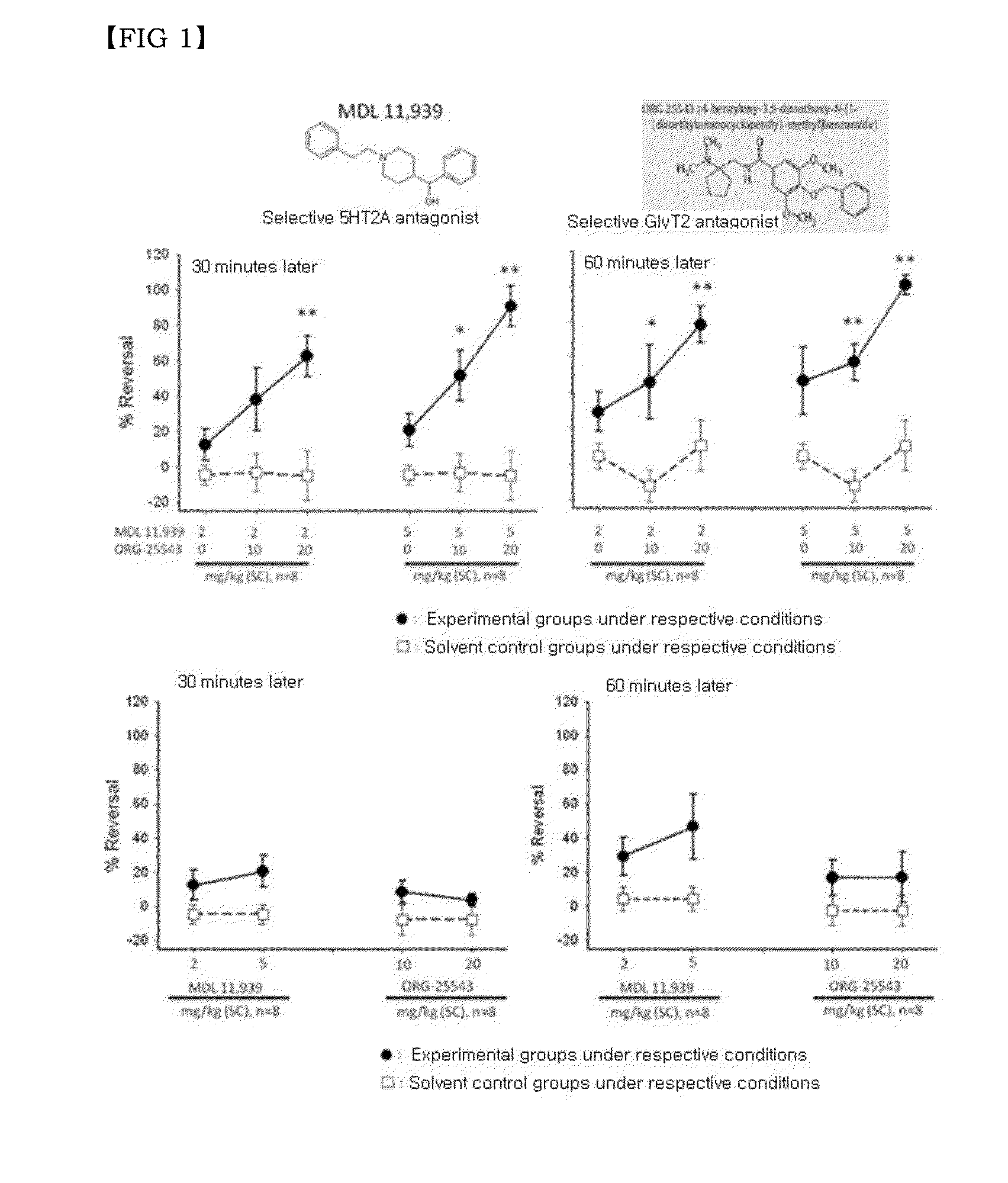
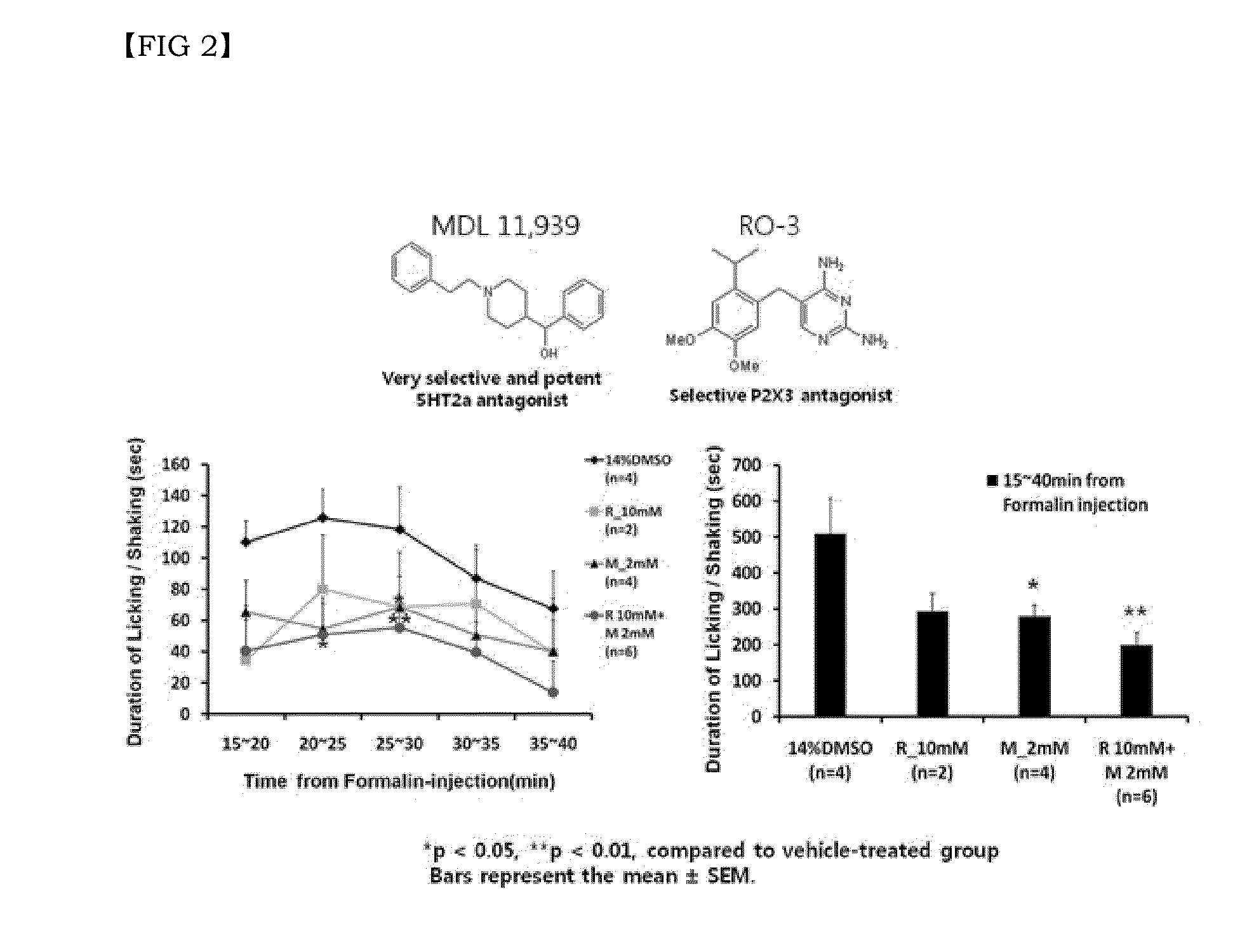
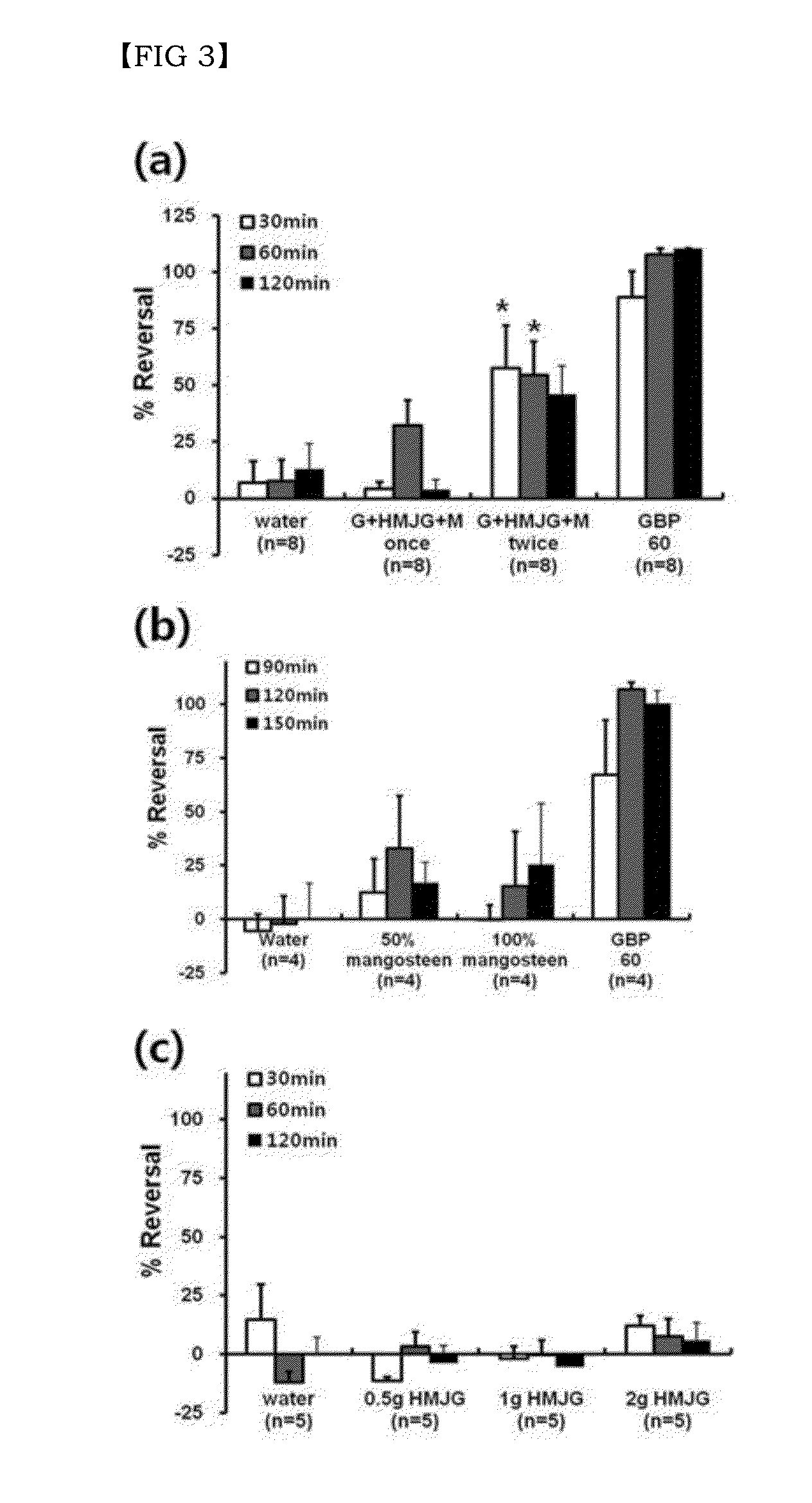
Combination of effective substances causing synergistic effects of multiple targeting and use thereof
InactiveUS20150290181A1Good curative effectPrevention and improvementBiocideCosmetic preparationsWrinkle skinDisease
Disclosed are a combination of active components inducing synergistic effects of multi-targeting and a use thereof. More particularly, disclosed are a functional food composition, a cosmetic composition, a pain-suppressive composition, and a composition for treatment or prevention of pruritus or atopic dermatitis, which comprise as active components, two or more components selected from a group consisting of (a) a 5-hydroxytryptamine subtype 2 (5-HT2) receptor antagonist; (b) a P2X receptor antagonist; and (c) any one of a glycine receptor agonist, a glycine transporter (GlyT) antagonist, a gamma-aminobutyric acid (GABA) receptor agonist, and a GABA transporter 1 (GAT1) antagonist. The multi-targeting composite composition (specifically, natural substances composite composition) has synergistic effects, and thus a treatment using a combination of respective components may achieve increased biological effects, in which mechanisms targeted by respective components are involved. Thus, the multi-targeting composite composition may not only remarkably control pain but may also increase effects of: alleviating symptoms of skin diseases such as itching and atopic dermatitis; preventing or improving depression, refreshment, pore minimization, improving wrinkles, skin regeneration, skin health, recovery of skin condition, skin whitening, preventing or improving athlete's foot, recovery of scalp health and regeneration of scalp, promoting hair growth, preventing gray hair, and improving dental and periodontal diseases, etc.
Owner:VIVOZON
Controlled release oxycodone compositions
InactiveUS20070275065A1Improve efficiencyQuality improvementBiocidePill deliveryControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately. 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean,of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour): administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +2
Targeted delivery of glycine receptors to excitable cells
ActiveUS20110213017A1Nervous disorderCell receptors/surface-antigens/surface-determinantsGlycine receptorAllosteric modulator
The invention provides a method of modulating electrophysiological activity of an excitable cell. The method involves causing exogenous expression of a glycine receptor (GlyR) protein in an excitable cell of a subject. Thereafter, the excitable cell is exposed to an allosteric modulator of the GlyR protein. Modulation of the exogenous GlyR protein (an ion channel) in response to the allosteric modulator modulates the electrophysiological activity of the excitable cell. The method can be used to control pain in a subject. The invention further provides a replication-defective HSV vector comprising an expression cassette encoding a GlyR protein, stocks and pharmaceutical compositions containing such vectors, and a transgenic animal.
Owner:UNIVERSITY OF PITTSBURGH
Compositions and methods for controlling pain
ActiveUS20160199663A1Relieve painNervous disorderPeptide/protein ingredientsControlling painControlled pain
The present disclosure provides compositions and methods for controlling pain. The present disclosure provides methods for identifying agents that control pain.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Extended release analgesic for pain control
InactiveUS6939538B2Electrostatic shieldingAvoid accessSalicyclic acid active ingredientsBiocideControlled releaseAbdominal cavity
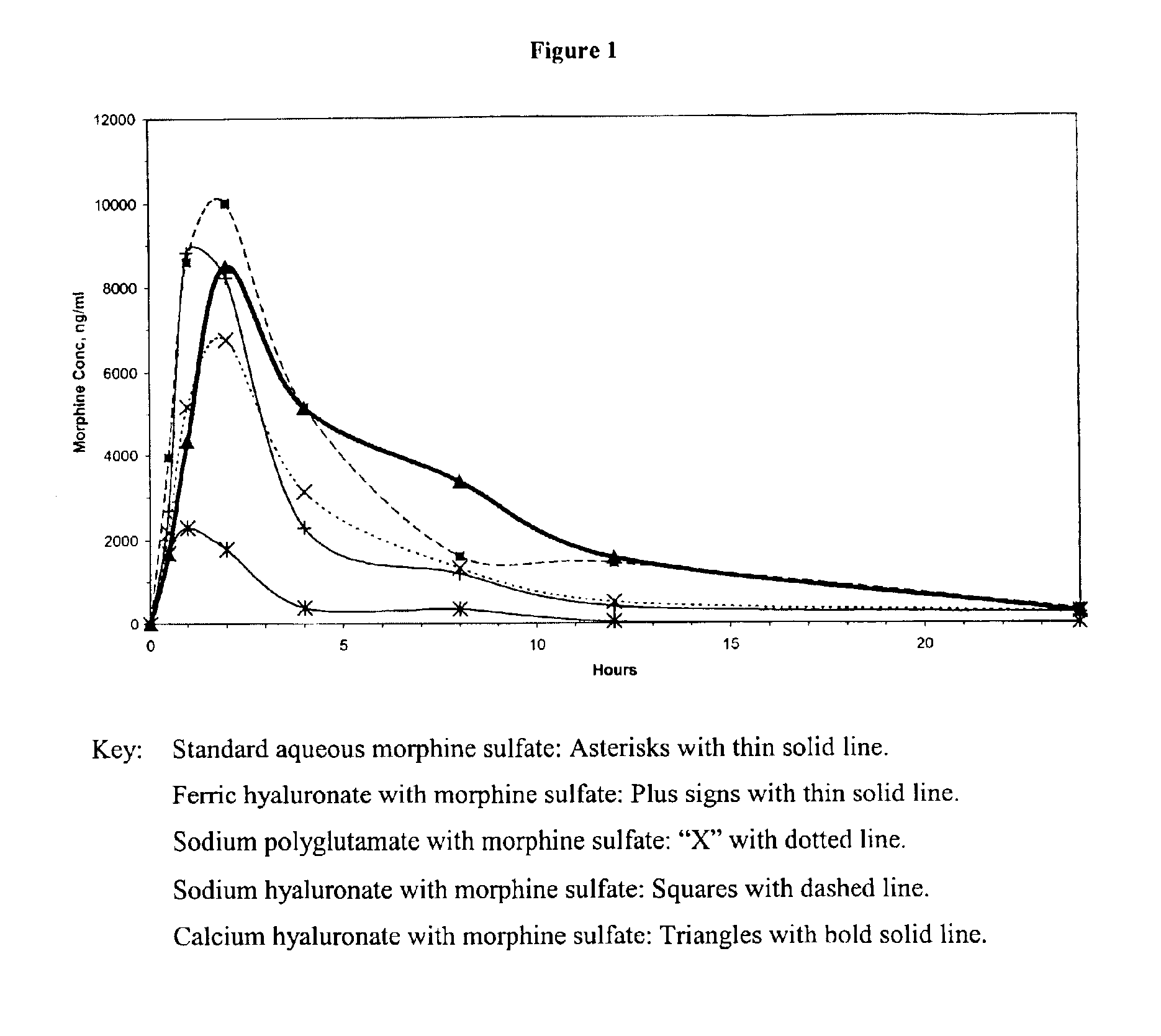
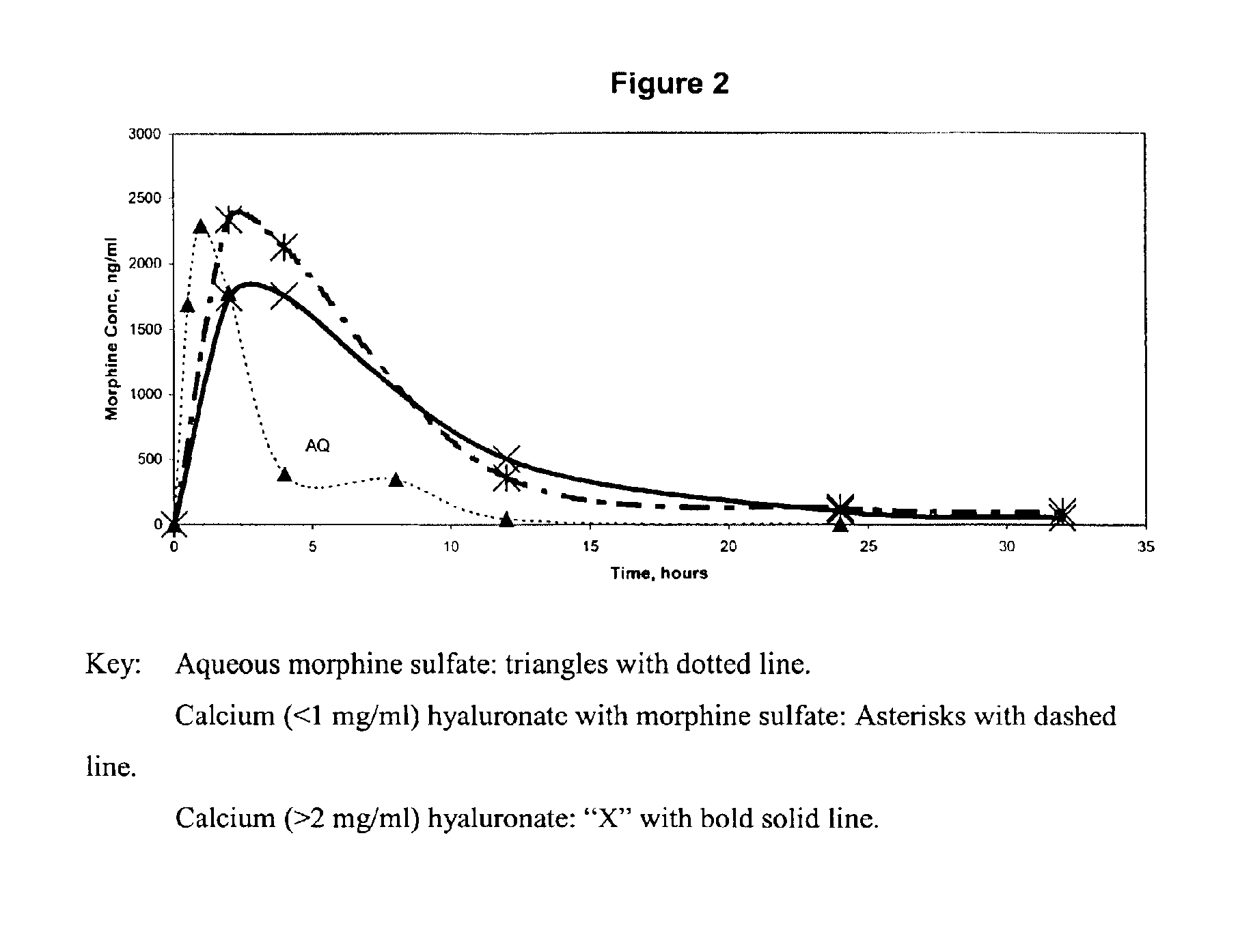
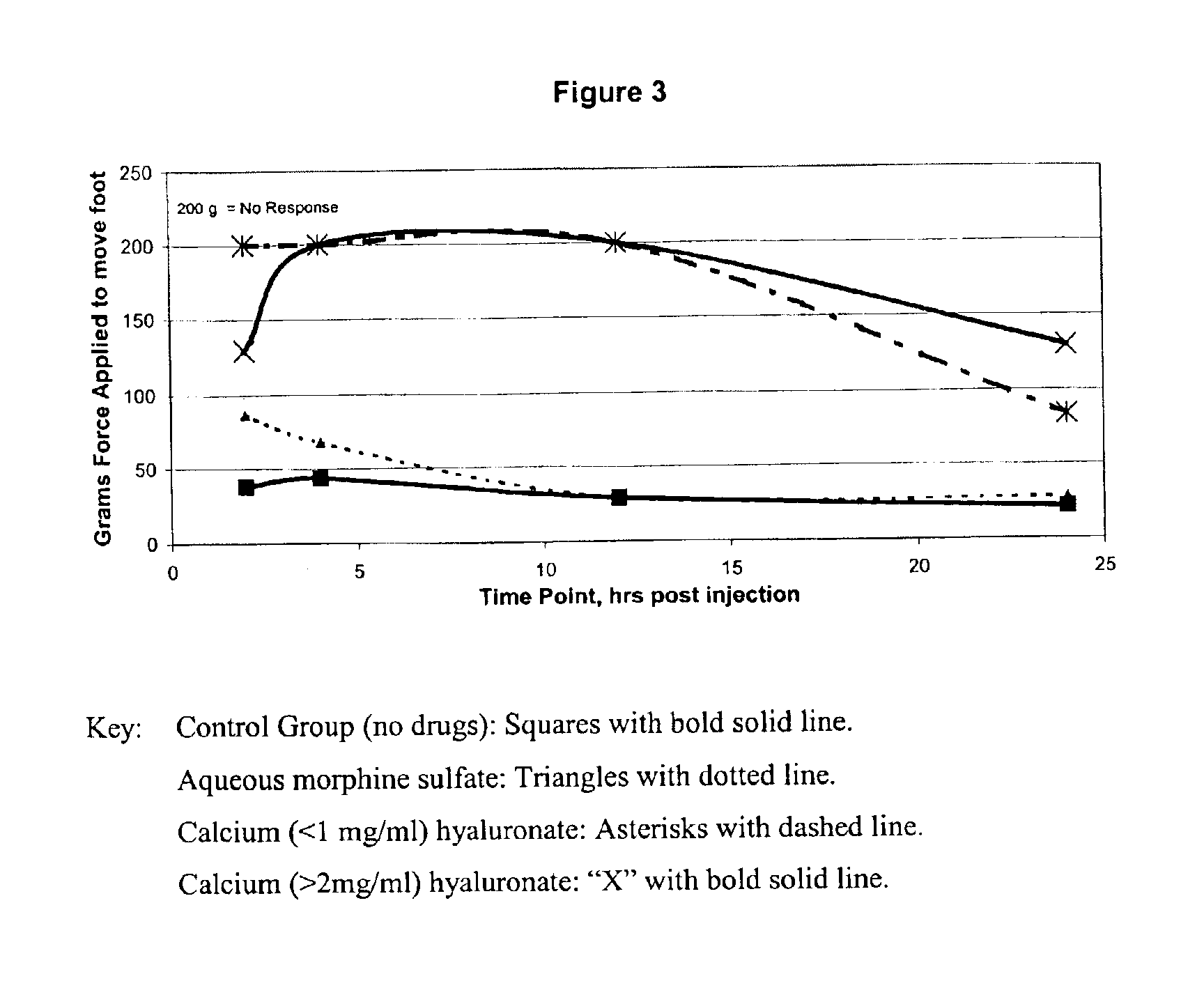
An extended release analgesic for controlling pain comprised of an opioid or non-opioid analgesic drug ionically bound to hyaluronic acid, poly-γ-glutamic acid or other ionic polymers, and injected into a body either subcutaneously, intramuscularly or intraperitoneally, utilizing counter-ions of different valences to control the rate of release into the body.
Owner:BIOMEDICAL RES MODELS
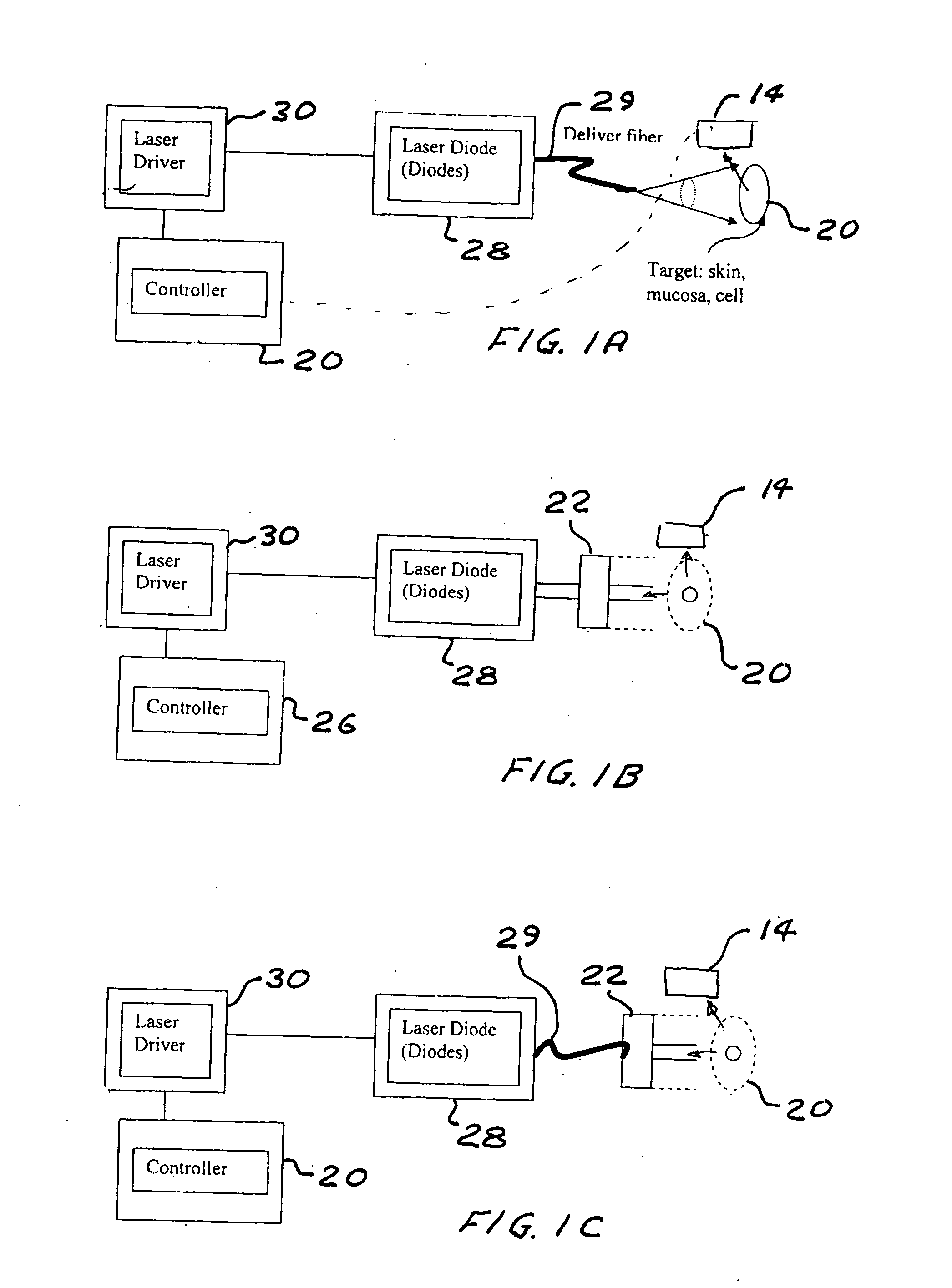
Portable laser and process for producing controlled pain
ActiveUS20050027336A1Avoid disagreementReduce beam divergenceDiagnostics using lightSurgical instrument detailsElectrophysiology studySingle type
A process and laser system for in vitro and in vivo pain research, pain clinical testing and pain management. In preferred embodiments of the present invention a diode laser operating at a 980 nm wavelength is used to produce warmth, tickling, itching, touch, burning, hot pain or pin-prick pain. The device and methods can be used for stimulation of a single nerve fiber, groups of nerve fibers, nerve fibers of single type only as well as more the one type of nerve fibers simultaneously. The present invention is especially useful for research of human / animal sensitivity, pain management, drug investigation and testing, and psychophysiology / electrophysiology studies. The device and methods permit non-contact, reproducible and controlled tests that avoid risk of skin damage. Applicant an his fellow workers have shown that tests with human subjects with the process and laser of the present invention correlate perfectly with laboratory tests with nerve fibers of rats. The device and the methods can be applied in a wide variety of situations involving the study and treatment of pain. Preferred embodiments of the present invention provide laser systems and techniques that permit mapping and single mode activation of C fibers and A-delta fibers.
Owner:NEMENOV MIKHAIL
Fixing device for experiment of stimulates of senses of pain and heat
InactiveCN1915186AAvoid damageSolve the problem of damage fixationDiagnostic recording/measuringSensorsNMR - Nuclear magnetic resonanceControlling pain
A fixing apparatus for the remotely controlled pain sense / warmth sense experiment of rat or mouse in functional nuclear magnetic resonance imaging environment is composed of animal fixer, coil fixer, beater for pain sense, and stimulating box for wormth sense.
Owner:徐杰 +1
Myocardial ischemia therapeutic instruments
ActiveCN101239222APayloadAddressing Adaptive IssuesArtificial respirationFrequency spectrumSpinal cord
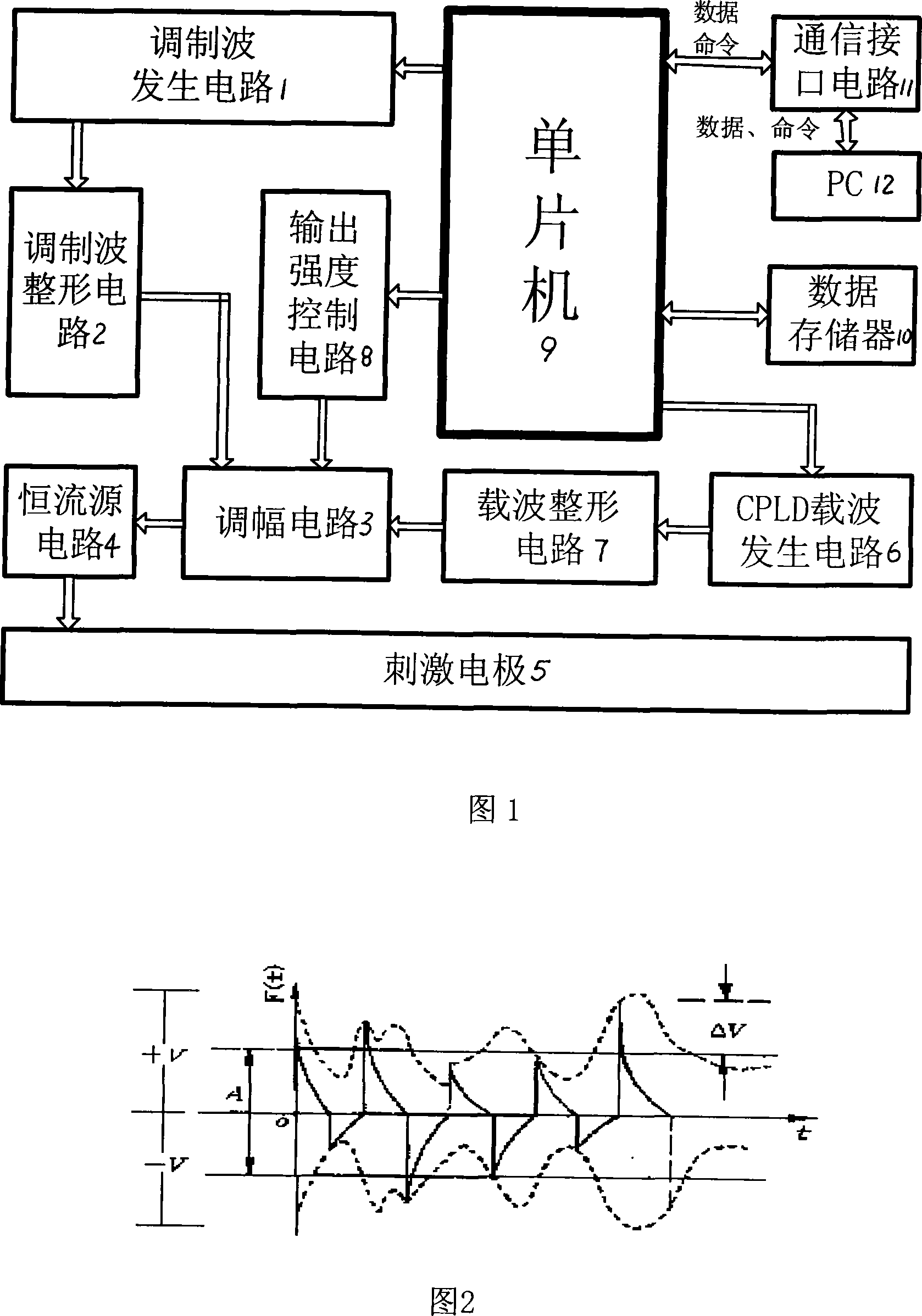
The present invention provides a therapy instrument for myocardial ischemia, which is combined with power circuit, generation circuit of modulation wave, shaping circuit of modulation wave, modulation circuit, constant current source circuit, stimulating electrode, CPLD carrier generation circuit, carrier shaping circuit, output intensity control circuit and MCU. The therapy instrument has characters of non-invasive, non-pain sense electrical stimulation, overcoming body adaptability etc., and can treat patient with comfortable sense. The therapy instrument can assistant treat myocardial ischemia by skin spinal cord and fastigial nucleus electrical stimulation. The therapy instrument modulates the random signal of low-frequency slow change rhythm on the MF carrier signal with special spectrum structure by method of pulse amplitude modulation, and loads LF current to human skin spinal cord and fastigial nucleus with forms of current by surface electrode. The therapy instrument can treat myocardial ischemia by improving coronary blood flow, reducing myocardial oxygen consumption, controlling pain and protecting neurogenic neuroprotection.
Owner:CHONGQING UNIV +1
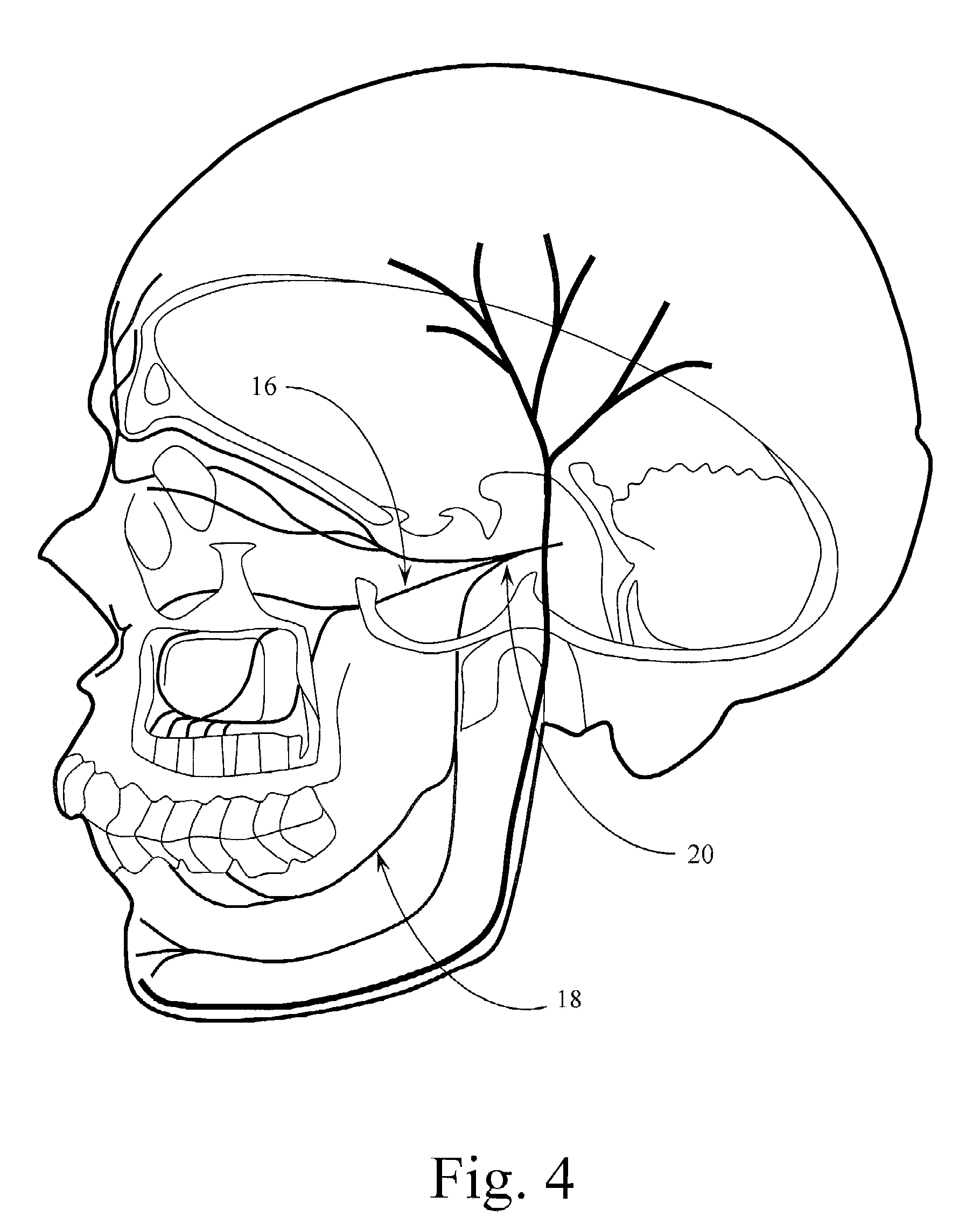
Trigeminal Nerve Stimulation Systems and Methods of Use
InactiveUS20110022126A1Increasing neural plasticitySpinal electrodesHead electrodesTrigeminal nerve stimulationNerve cells
Owner:WAYKI
Traditional Chinese medicine compound pain resisting medicine and preparation method thereof
InactiveCN104606324AAddress Pain ConditionsAvoid painNervous disorderAnthropod material medical ingredientsSide effectDeep frying
The invention relates to a traditional Chinese medicine for treating pain and a preparation method of the traditional Chinese medicine, in particular to a traditional Chinese medicine compound pain resisting medicine and a preparation method thereof. The recipe comprises the following materials: nux vomica, lumbricus, garden balsam stem, stiff silkworm, fourstamen stephania root, radix clematidis, Chinese angelica, raw rhubarb, herba lycopi, frankincense, myrrh, drynaria baronii, cowherb seed, asarum sieboldii, cortex acanthopanacis senticosus, herba siegesbeckiae, Chinese holly leaf, centipede, loofah sponge, ephedra and the like. The preparation method comprises the following steps: taking 2,000 ml of sesame oil into a pot, putting the materials into the pot, carrying out deep frying, removing slag, refining oil, dropping water to be bead, feeding 1,000 g of minium, and stirring. Through the adoption of traditional Chinese medicine ointments and medicines for external use, the medicine resistance and toxic and side effects generated after oral taking of Western medicines or chemical agents are solved, pain and inconvenience brought for a patient due to injection treatment are avoided, the pain symptoms of the patient are solved, the pain of the patient is reduced, and the functions of warming channel, dredging collaterals, relaxing muscles, stimulating blood circulation and stopping pain can effectively control pain seizure.
Owner:刘艳军
Selective ablation of pain-sensing neurons by administration of a vanilloid receptor agonist
The present invention provides methods and kits for the selective ablation of pain-sensing neurons. The methods comprise administration of a vanilloid receptor agonist to a ganglion in an amount that causes death of vanilloid receptor-bearing neurons. Accordingly, the present invention provides methods of controlling pain and inflammatory disorders that involve activation of vanilloid receptor-bearing neurons.
Owner:UNITED STATES OF AMERICA
Controlled release oxycodone compositions
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:OSHLACK BENJAMIN +3
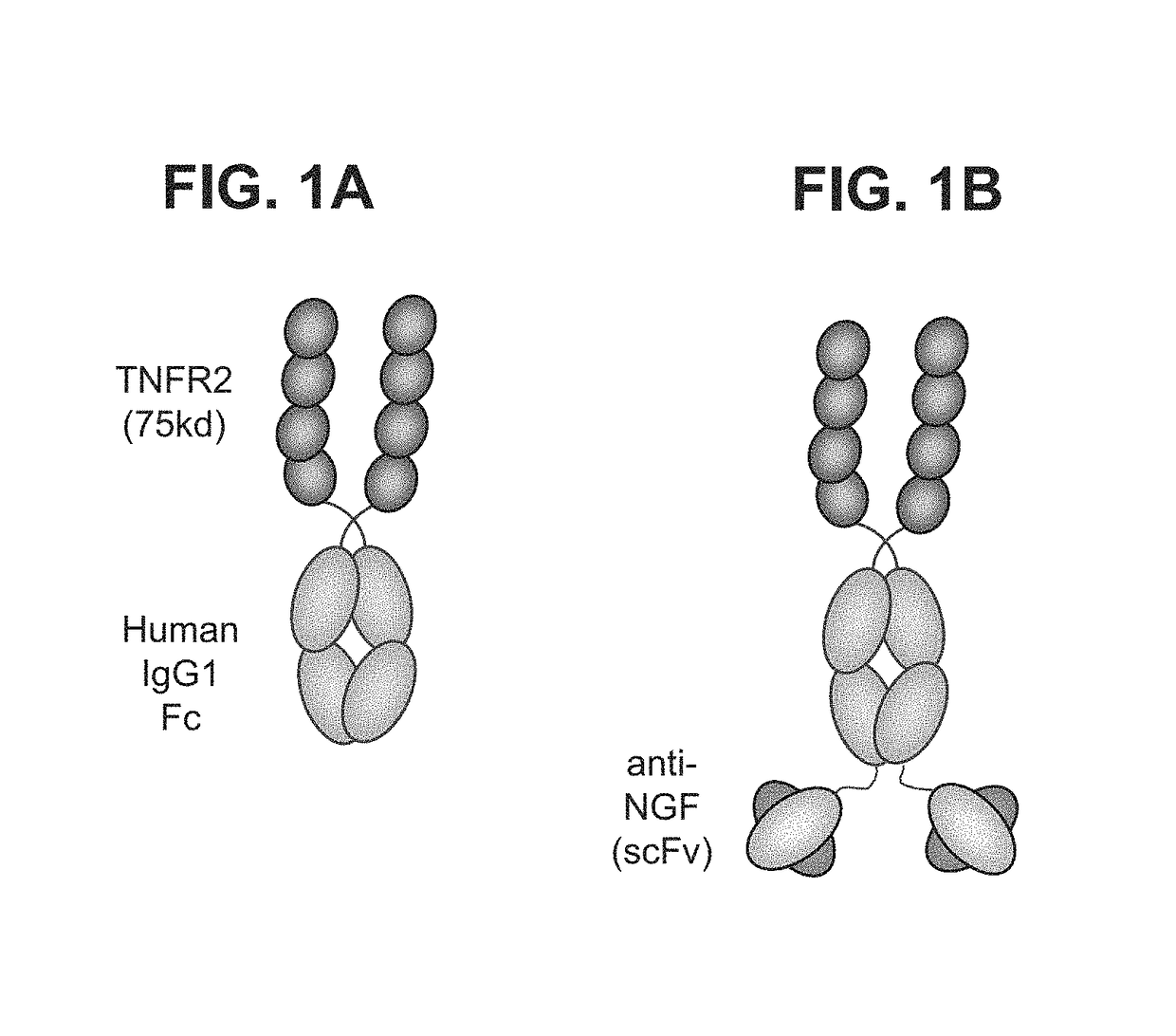
Compounds and methods for treating pain
ActiveUS9884911B2Prevent and reduce and ameliorate and eliminate painPain controlNervous disorderAntipyreticCo administrationTnfα antagonist
Owner:MEDIMMUNE LTD
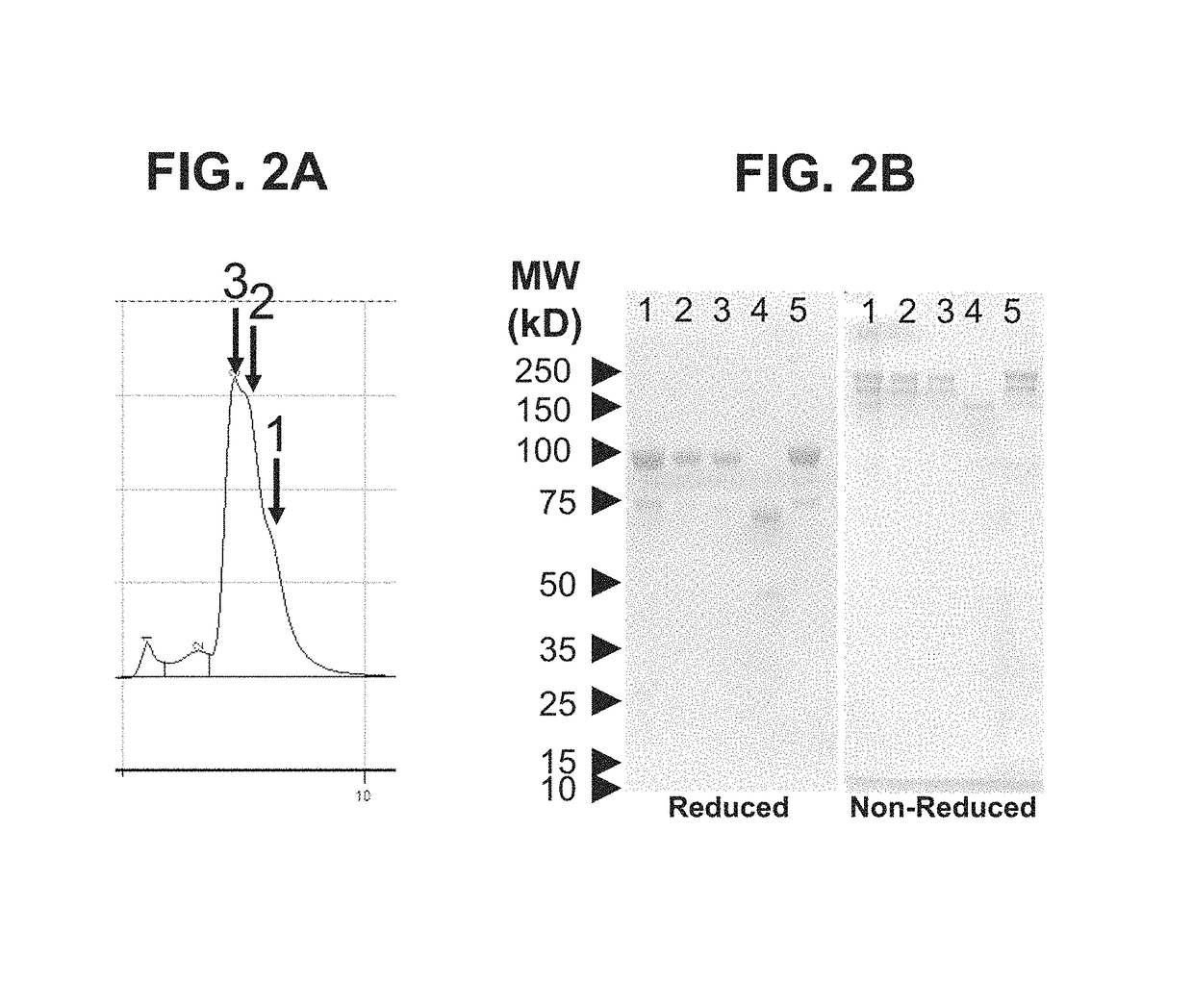
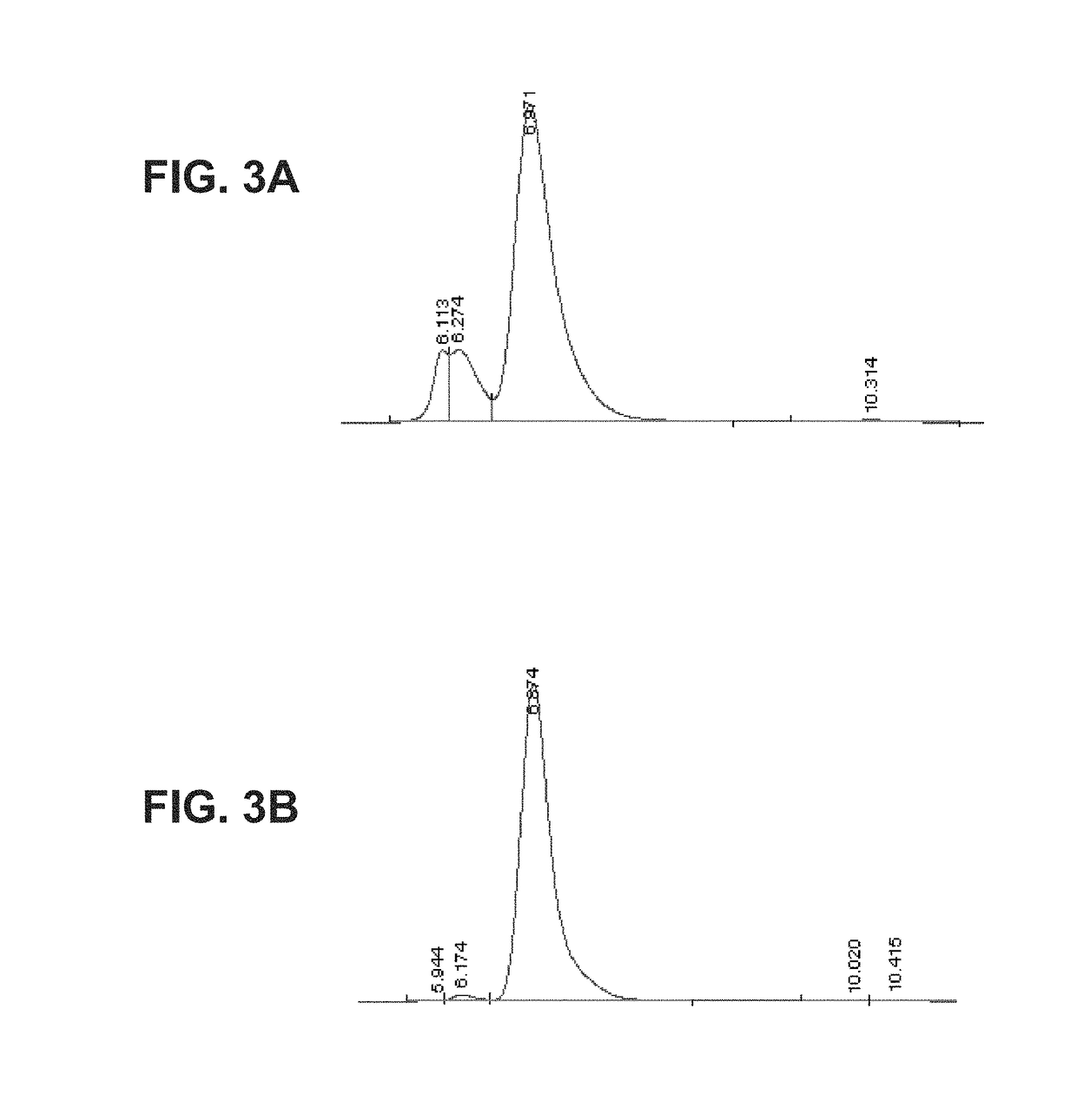
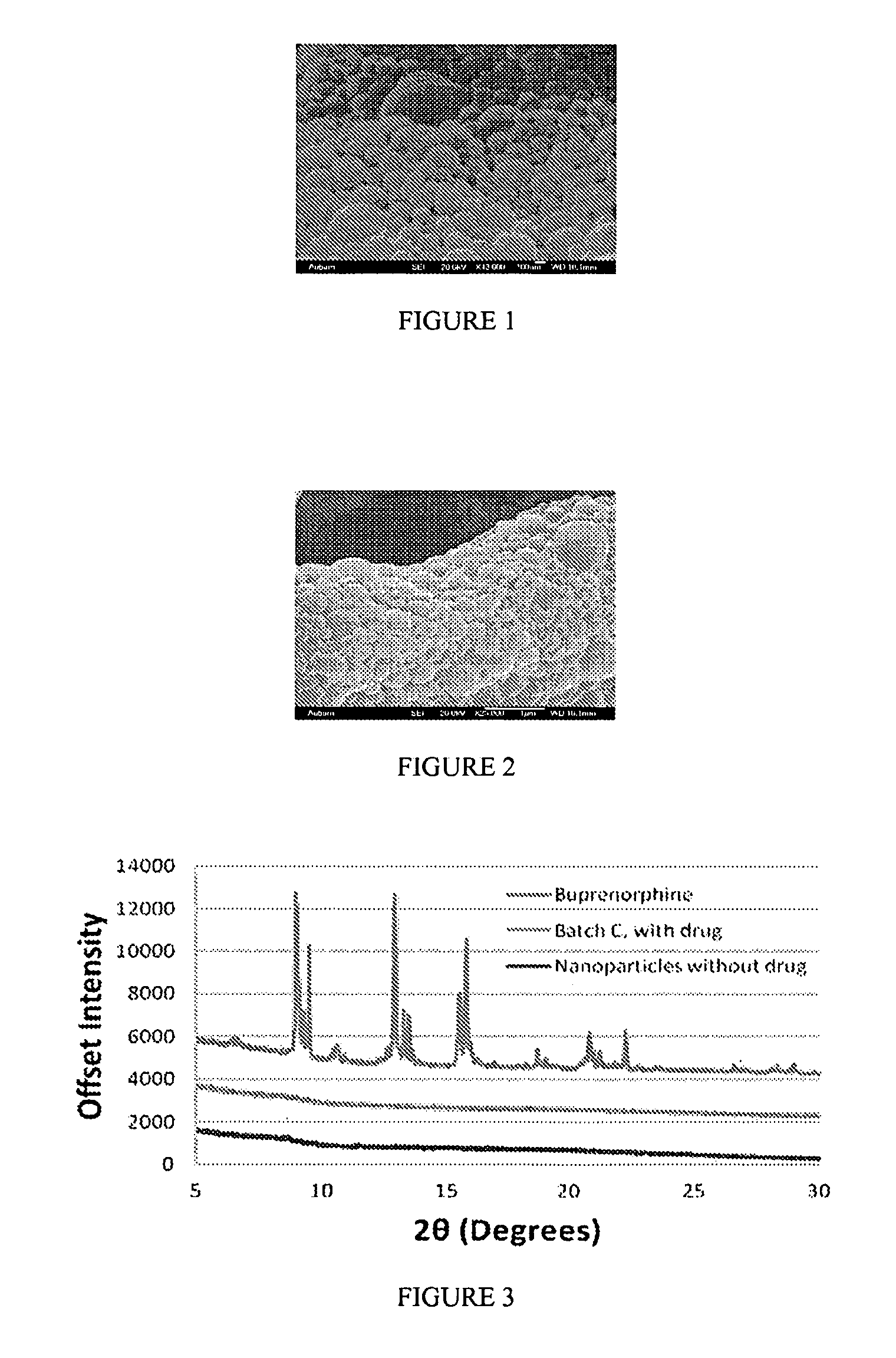
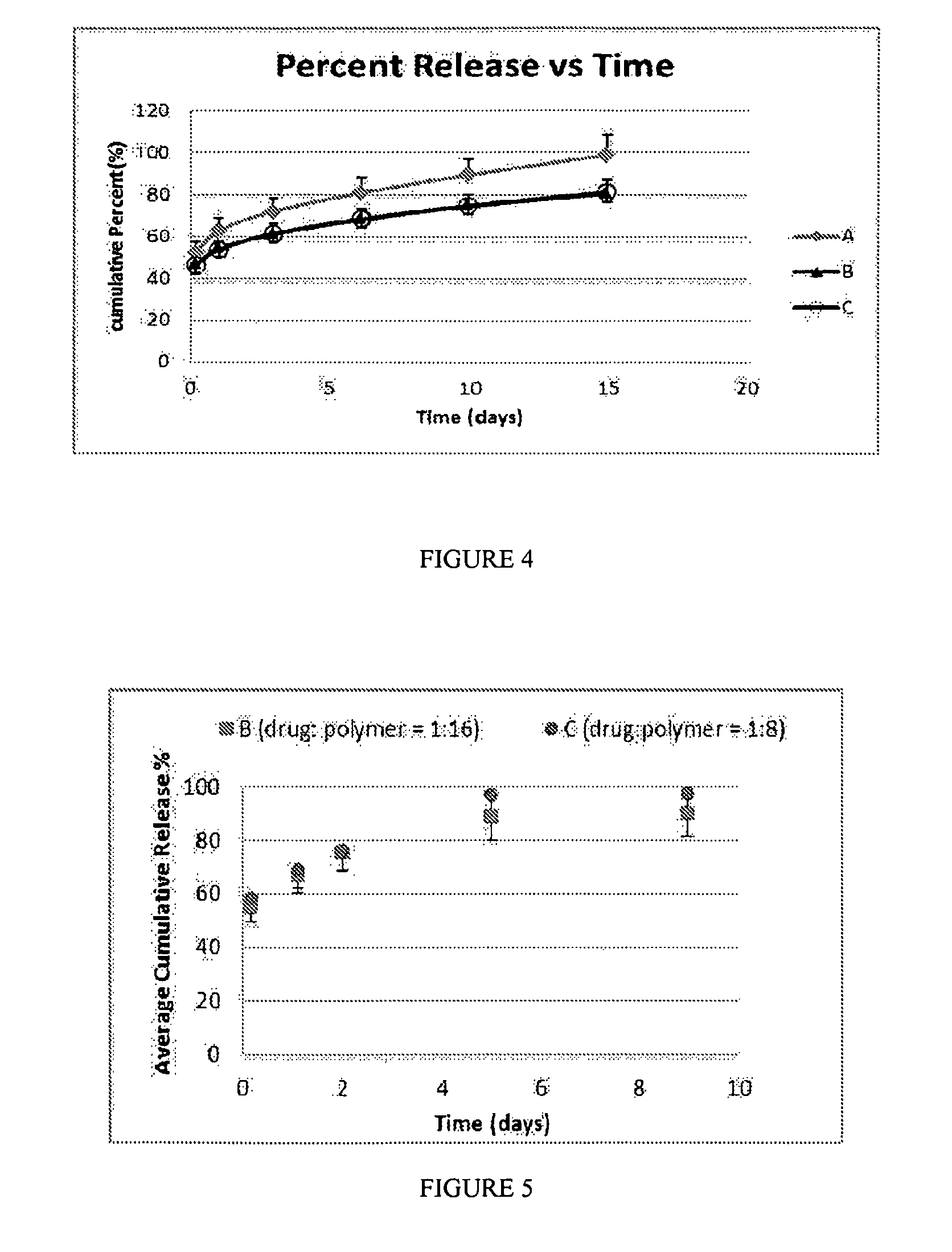
Buprenorphine nanoparticle composition and methods thereof
ActiveUS9566241B2Prone to abuseLow absorptionOrganic active ingredientsGranular deliverySurgical removalParticle composition
Owner:AUBURN UNIV
Selective Ablation of Pain-Sensing Neurons by Administration of a Vanilloid Receptor Agonist
The present invention provides methods and kits for the selective ablation of pain-sensing neurons. The methods comprise administration of a vanilloid receptor agonist to a ganglion in an amount that causes death of vanilloid receptor-bearing neurons. Accordingly, the present invention provides methods of controlling pain and inflammatory disorders that involve activation of vanilloid receptor-bearing neurons.
Owner:THE GOV OF THE US REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICE
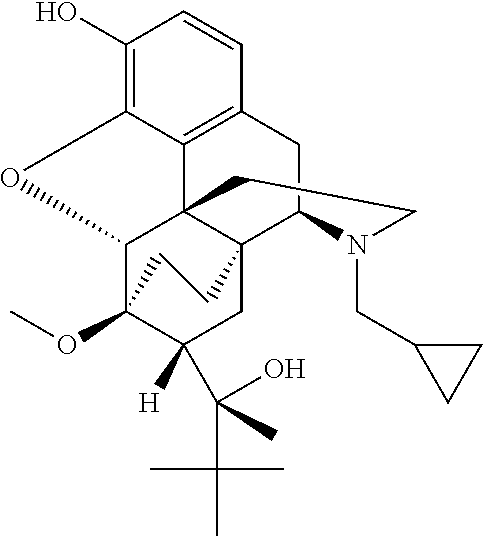
Opioid delivery system
An opioid formulation for pulmonary administration in the treatment or management of pain, a pulmonary drug delivery device containing, method of administering, kit containing, and uses of same. The formulation contains at least one rapid-onset opioid and preferably also contains a sustained-effect opioid to reduce the frequency of administration. The invention employs the side effects of the opioid formulation to permit patients to self-limit drug intake, thereby avoiding toxicity while achieving analgesia. A pharmacokinetic and pharmacodynamic model is employed to determine optimum drug formulations and optimum parameters for administration.
Owner:YM BIOSCI
Non-invasive method for prediction of opioid-analgesia and opioid-blood-concentrations
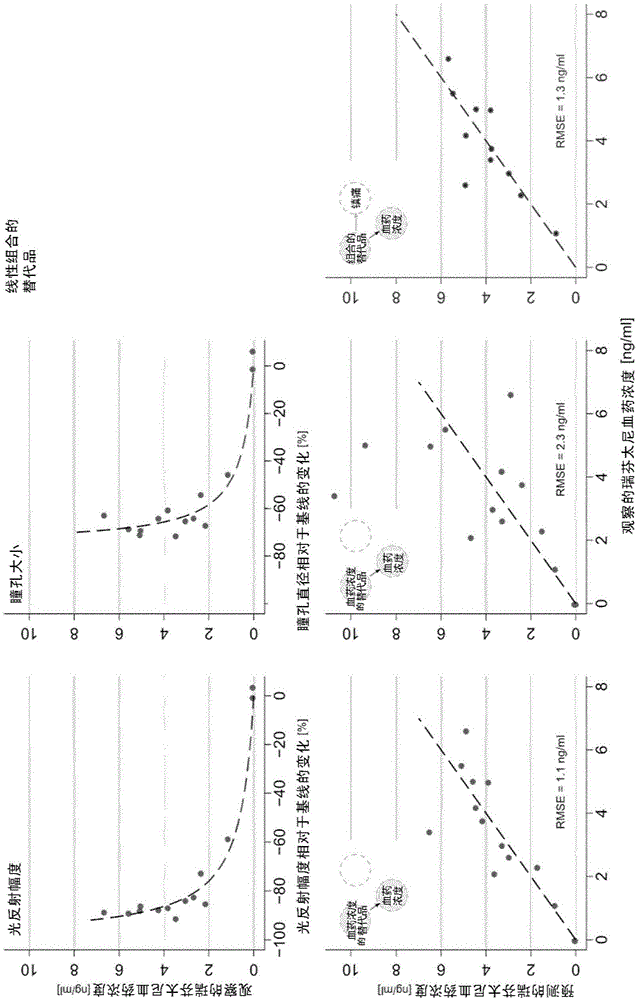
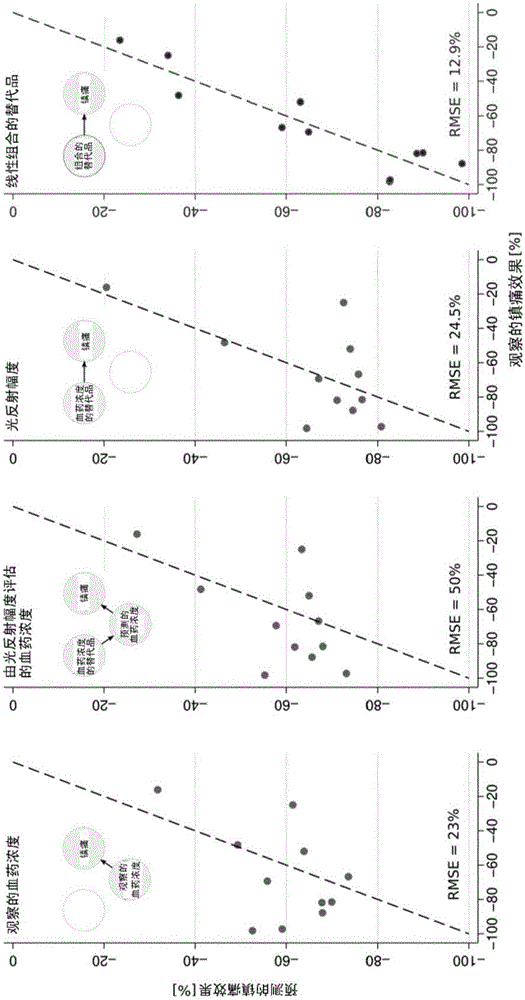
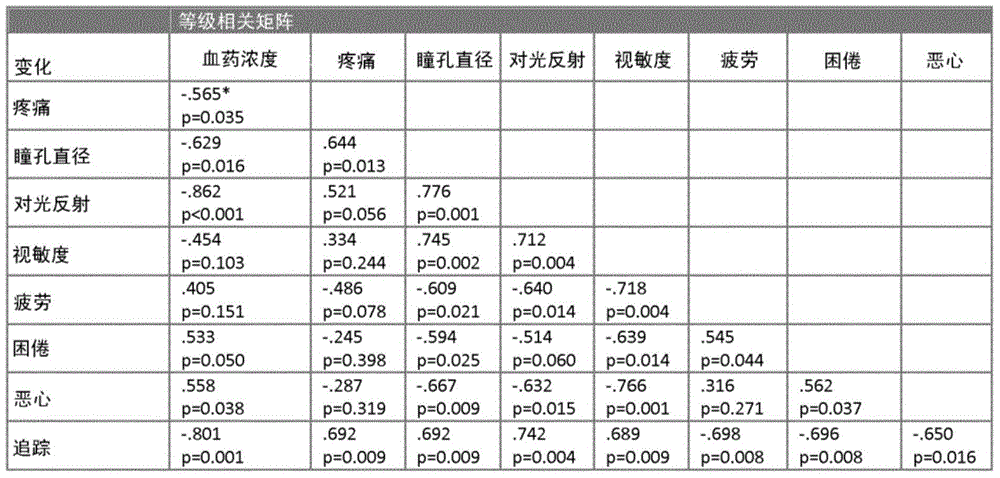
The present invention pertains to a non-invasive method and apparatus for predicting or monitoring analgesia and blood levels of opioid drugs in a patient receiving pain treatment, e.g., during palliative treatment. The inventive method comprises the measurement of one or more, preferably two or more, surrogate markers of a patient. According to the present invention surrogate markers correlate with the level of analgesia in the opioid receiving subject and thus provide a non-invasive method to predict and monitor analgesia during a treatment. Even more, surrogate markers were identified which correlate with the blood-concentration of the opioid in the subject. Thus, the invention provides a valuable clinical tool to assess and control pain treatments with opioids. Disclosed is the prediction method, an apparatus suitable for performing the inventive methods as well as the apparatus for use in medical treatments, such as pain therapy.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com