Industrialized synthesis method of medicinal indocyanine green
A technology of indocyanine green and synthetic method, which is applied in the field of pharmacy, can solve the problems of many reaction steps, inconvenient industrial production, and low product yield, and achieve the effects of less synthetic reaction steps, easy production operation, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
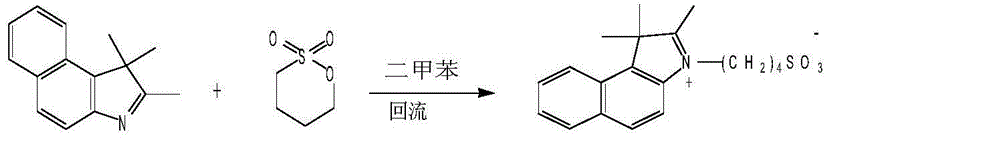
[0017] 1) Synthesis of 2,3,3-trimethyl-1-(4-sulfobutyl)-4,5-benzindole betaine:
[0018]
[0019] Specifically: mix 2,3,3-trimethyl-4,5-benzindolebenzene and 1,4-butyl sultone at a weight ratio of 1:1.6, in the presence of xylene Reflux at 140°C for 3 hours, cool to 50°C and add acetone 6 times the volume of xylene, reflux at 55°C for 0.5 hour, cool to 20°C, filter and dry. That is, light blue solid 2,3,3-trimethyl-1-(4-sulfobutyl)-4,5-benzindole betaine, wherein, according to 2,3,3-trimethyl Base-4,5-benzindolebenzene 1:0.8 weight ratio was added to xylene.
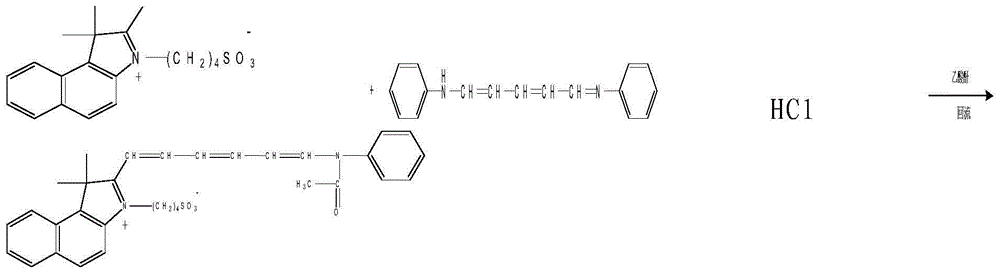
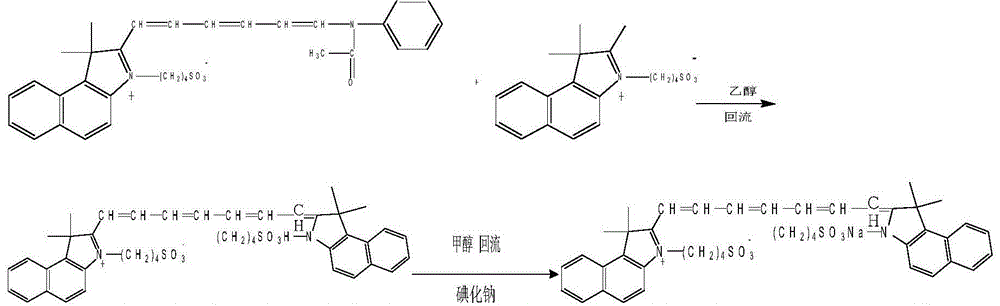
[0020] 2) 2-[6-(N-ethylanilino)-1,3,5-hexatrien-1-yl]-3,3-dimethyl-1-(4-sulfobutyl)-4 , Synthesis of 5-benzindole betaine
[0021]
[0022] Specific process: the above-mentioned obtained 2,3,3-trimethyl-1-(4-sulfobutyl)-4,5-benzindole betaine and 2-pentenedial dianiline hydrochloride Salts were mixed at a weight ratio of 1:0.9, refluxed and condensed at 140°C for 5 minutes in the presence of acetic anhydride, t...
Embodiment 2
[0029] 1) Synthesis of 2,3,3-trimethyl-1-(4-sulfobutyl)-4,5-benzindole betaine:
[0030]
[0031]Specifically: mix 2,3,3-trimethyl-4,5-benzindolebenzene and 1,4-butyl sultone at a weight ratio of 1:1.7, in the presence of xylene Reflux at 142°C for 2 hours, cool to 60°C, add acetone 4 times the volume of xylene, reflux at 52°C for 0.5 hour, cool to 25°C, and filter and dry. That is, light blue solid 2,3,3-trimethyl-1-(4-sulfobutyl)-4,5-benzindole betaine, wherein, according to 2,3,3-trimethyl Base-4,5-benzindolebenzene 1:1.2 weight ratio was added to xylene.
[0032] 2) 2-[6-(N-ethylanilino)-1,3,5-hexatrien-1-yl]-3,3-dimethyl-1-(4-sulfobutyl)-4 , Synthesis of 5-benzindole betaine
[0033]
[0034] Specific process: the above-mentioned obtained 2,3,3-trimethyl-1-(4-sulfobutyl)-4,5-benzindole betaine and 2-pentenedial dianiline hydrochloride The salts were mixed in a weight ratio of 1:1.1, refluxed and condensed at 130°C for 10min in the presence of acetic anhydride, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com