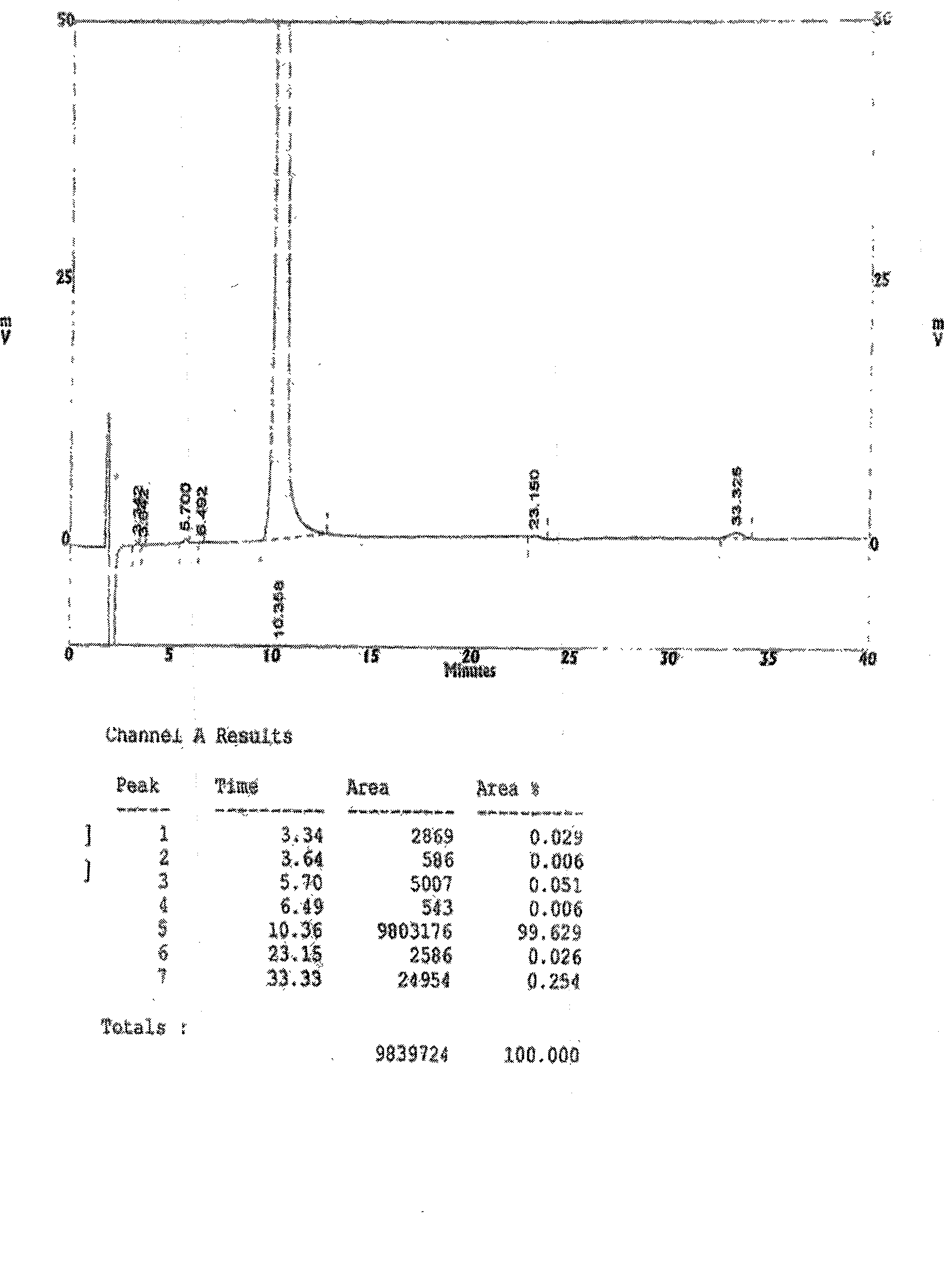
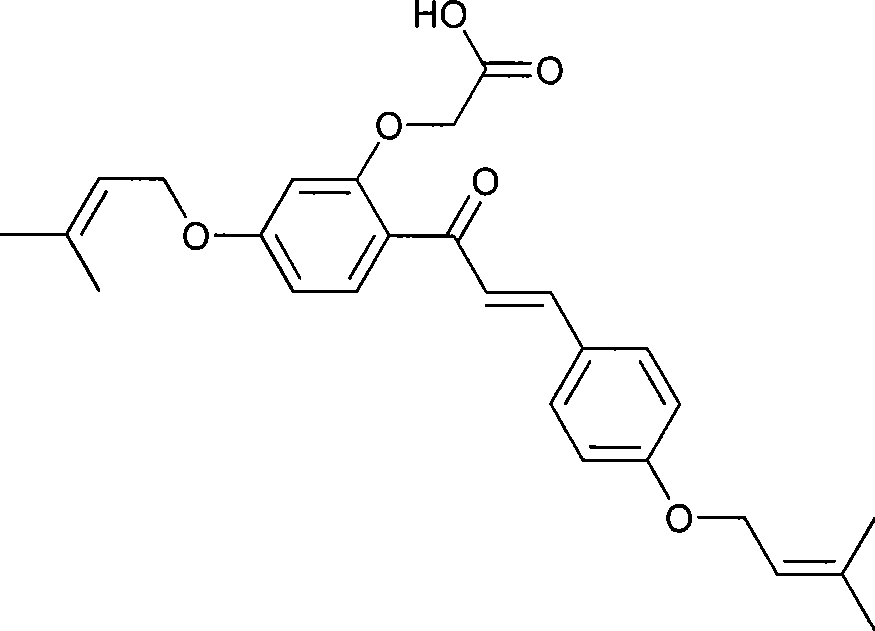
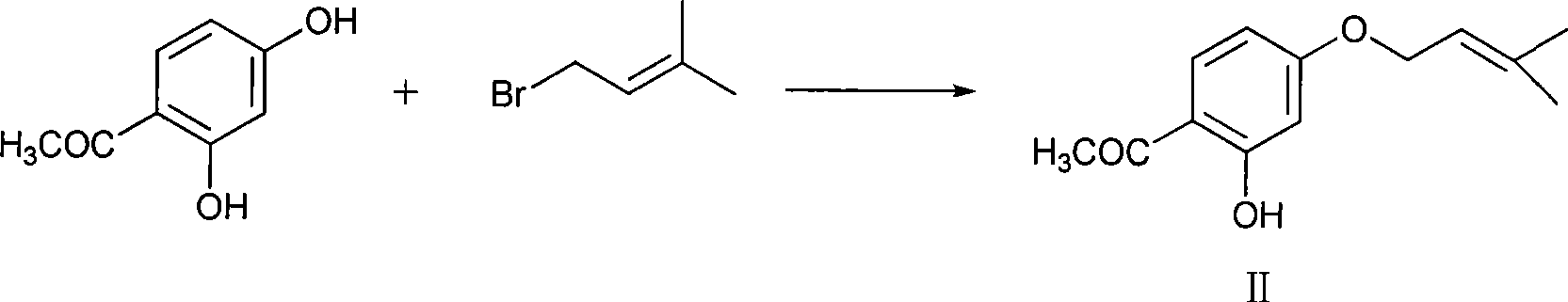
Novel preparation of sofalcone
A sofa ketone and a novel technology, applied in the field of sofa ketone preparation, can solve problems such as low yield and poor quality of intermediates, and achieve the effects of improving yield, ensuring quality and simplifying processing methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of 1-[2-hydroxyl-4-(3-methyl-2-butenyloxy)-phenyl]-3-(4-hydroxyphenyl)-propenone (IV)
[0038] In a 3000mL reaction flask equipped with stirring, condenser, thermometer and dropping funnel, add 200g of 2-hydroxy-4-(3-methyl-2-butenyloxy)acetophenone and 750mL of absolute ethanol, start stirring , Add 1250 mL of 10% potassium hydroxide aqueous solution. After the reaction system was dissolved, slowly add 27.7g p-hydroxybenzaldehyde. After the addition was complete, the reaction was continued for 1 hour, and then 27.7 g of p-hydroxybenzaldehyde was added. After the addition was completed, the reaction was incubated at 45°C for 5 hours.
[0039] Add 400 mL of acetonitrile, stir and cool down to 15°C, adjust the pH of the reaction solution to 5-6 with 18% aqueous hydrochloric acid, and a yellow solid precipitates. Filter, wash the filter cake once with 50mL of cold absolute ethanol, once with 50mL of petroleum ether, and dry to obtain 136.85g of a ye...
Embodiment 2
[0040] Example 2: Synthesis of 1-[2-hydroxyl-4-(3-methyl-2-butenyloxy)-phenyl]-3-(4-hydroxyphenyl)-propenone (IV)
[0041]In a 3000mL reaction flask equipped with stirring, condenser, thermometer and dropping funnel, add 200g of 2-hydroxy-4-(3-methyl-2-butenyloxy)acetophenone and 750mL of absolute ethanol, start stirring , Add 1450 mL of 10% aqueous sodium hydroxide solution. After the reaction system was dissolved, slowly add 55g of p-hydroxybenzaldehyde. After the addition was complete, the reaction was continued for 1.5 hours, and then 55g of p-hydroxybenzaldehyde was added. After the addition was completed, the reaction was incubated at 25°C for 10 hours.
[0042] Add 400 mL of acetonitrile, stir and cool down to 15°C, adjust the pH of the reaction solution to 5-6 with 18% aqueous hydrochloric acid, and a yellow solid precipitates. Filter, wash the filter cake once with 50mL of cold absolute ethanol, once with 50mL of petroleum ether, and dry to obtain 234.62g of a yell...
Embodiment 3
[0043] Example 3: Synthesis of 1-[2-hydroxyl-4-(3-methyl-2-butenyloxy)-phenyl]-3-(4-hydroxyphenyl)-propenone (IV)
[0044] In a 3000mL reaction flask equipped with stirring, condenser, thermometer and dropping funnel, add 200g of 2-hydroxy-4-(3-methyl-2-butenyloxy)acetophenone and 750mL of absolute ethanol, start stirring , Add 1650 mL of 10% potassium hydroxide aqueous solution. After the reaction system was dissolved, slowly add 138.6g p-hydroxybenzaldehyde. After the addition was complete, the reaction was continued for 2 hours, and then 138.6 g of p-hydroxybenzaldehyde was added. After the addition was completed, the reaction was incubated at 5°C for 15 hours.
[0045] Add 400 mL of acetonitrile, stir and cool down to 15°C, adjust the pH of the reaction solution to 5-6 with 18% aqueous hydrochloric acid, and a yellow solid precipitates. Filter, wash the filter cake once with 50mL of cold absolute ethanol, once with 50mL of petroleum ether, and dry to obtain 270.0g of a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com